Fish immune system—an overview
Like mammals, the fish immune system is built with two components, i.e., non-specifc, or innate immunity, and specifc or adaptive immunity. The nonspecifc immune system in fish is the second line of defence, which responds non-specifcally against pathogens before the specifc immune system takes command of the defence mechanism (Firdaus-Nawi and Zamri-Saad 2016). Both specifc and non-specifc immune responses are complementary to each other to prevent infection in fish. In this section, we will discuss fish immunity with a focus on teleost fishes (largest infraclass of freshwater ray fnned bony fishes).
The components of the non-specifc immune system such as mucus and epithelial cells that line the skin, gills, and stomach provided a physical barrier. When pathogens breach the physical barriers, the components of non-specifc cellular immune defence cells, such as monocytes, macrophages, and granulocytes (neutrophils and eosinophilic granulocytes), are involved to the invaded infamed site and neutralize the microbes by using non-specifc molecular and cellular mechanisms (Corbel 1975; Havixbeck et al. 2016).
To prevent pathogenic growth, humoral components of the innate immune response attract diferent proteins and glycoproteins such as lysozyme, C-reactive proteins, lectins, interferons, and transferrin (Firdaus-Nawi and Zamri-Saad 2016). Leucocytes, including macrophages or monocytes, granulocytes, macrophages, and non-specifc cytotoxic cells (NCCs), toll-like receptors (TLRs), and dendritic cells are the main cell types involved in non-specifc cellular immunity.
They are produced from pluripotent stem cells in the lymphoid tissue of fish like head kidney, thymus, spleen, and gut-associated mucosal tissue (Biller-Takahashi and Urbinati 2014). The liver and head kidney are also involved in the production of innate humoral soluble acute phase molecules such as C-reactive protein, anti-protease, complement components, transferrin, lectin, and serum amyloid A. These acute phase proteins are an integral part of humoral innate response. These also act on the head kidney and increase the diferentiation of leukocytes (Firdaus-Nawi and Zamri-Saad 2016).
The superfcial part of fish such as gill, gut, gastrointestinal tract, and skin are the entry points for pathogenicity since they are in contact with the surroundings. Foreign stimuli are received primarily by these mucosal tissues and produce many substances which are messengers like small peptides, cytokines, and hormones that will initiate the overall physiological response, fostering the non-specifc immune response which occurs at systemic level (Gomez et al. 2013). The main cellular components of specifc immunity are B and T lymphocytes.
The B lymphocytes are developed in the head kidney of fish and are considered as primary lymphoid organ similar to the bone marrow of mammal (Parra et al. 2015).
Spleen is considered as secondary lymphoid organ because B lymphocytes are activated and diferentiated into plasma memory cells within it (Fig. 1A).
The connection between non-specifc and specifc immunity is mediated by macrophages and dendritic cells as they respond to pathogens to activate T lymphocytes using the class-II MHC (Fig. 1B). The maturation of T cells takes place in the thymus of fish (Biller-Takahashi and Urbinati 2014; Firdaus-Nawi and Zamri-Saad 2016).
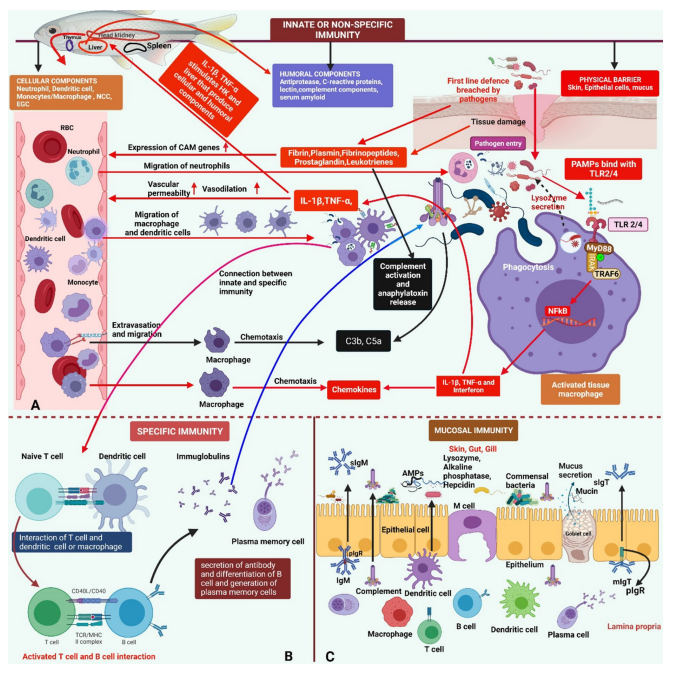
Fig. 1 Fish immunity overview:
A Non-specifc immunity: Entry, tissue damage and releasing of fbrin, plasmin, fbrinopeptides, prostaglandins, leukotrienes from infected tissue increases extravasation and migration of dendritic cells, macrophages, neutrophils through elevating vasodilation and vascular permeability. PAMPs bind with TLR2/4 on tissue macrophage and activate TLR signalling intermediate molecules (MyD88, IRAK, TRAF6, NFκB) (Akira et al. 2006; Cao et al. 1996; Covello et al. 2009; Didonato et al. 1997; Schwandner et al. 1999; Wajant et al. 2003; Wesche et al. 1997) which sequentially induce the expression of chemokines, cytokines (IL-1β, TNF-β) (Firdaus-Nawi and Zamri-Saad 2016; Huising et al. 2004; Secombes et al. 2001; Whyte 2007). Chemokines and cytokines also do trafcking phagocytes and antigen presenting cells (APCs) as well as stimulate head kidney (HK) and liver to produce cellular and humoral components (BillerTakahashi and Urbinati 2014) (Red Box and arrows).Tissue macrophages also secrete lysozyme which disrupt bacterial cell wall (Saurabh and Sahoo 2008) (Dotted black arrow). Complement C3b and C5a are released from complement complex and are potent chemotoxin for migration of macrophages and neutrophils (Iwama et al. 1996) (Black box and arrows). C3b also help in phagocytosis of pathogens by macrophage (Bayne and Gerwick 2001; Boshra et al. 2006; Ellis 2001).
B Specifc immunity: macrophage, neutrophils (APCs) uptake pathogens from infected site and interact with T cells (Pink arrow) that in turn interact with B cells that produce immunoglobulins and immunoglobulins participate in formation of complement complex (Blue arrow).
C Mucosal immune components (Gill, Gut and Skin): cellular components-Dendritic cells, macrophages, epithelial cells, B cells, T cells, plasma cells, goblet cells and M cells. Humoral components: sIgM, sIgT, AMPs, pIgR, complements, alkaline phosphatase, hepcidin, lysozyme, mucin. PAMPs, pathogen-associated molecular patterns; TLR2/4, toll-like receptor 2/4; MyD88, myeloid diferentiation primary response 88; IRAK, Interleukin-1 Receptor-Associated Kinase; TRAF6, tumour necrosis factor (TNF) receptor-associated factor 6; NF-κB, Nuclear factor kappa B; IL-1β, Interleukin 1 beta; TNF-α, tumour necrosis factor-alpha; HK, head kidney; CAMs, Cell adhesion molecules; C3b, complement component 3b; C5a, Complement Factor 5a; NCCs, Non-specifc cytotoxic cells; EGCs, Eosinophilic granular cells; APCs, antigen presenting cells; RBC, red blood cells; sIgM, secreted immunoglobulin M; sIgT, secreted immunoglobulin T, AMPs, antimicrobial peptides; pIgR, polymeric immunoglobulin receptors
The process of fish immunity is not as complex as that of mammals, but it provides necessary protection against antagonistic pathogens from the environment. Most studies focus on haematopoiesis and cytokine generation to gain a better understanding of fish immunological health.
Haematopoiesis is essential for both innate and adaptive immunity, where the defence cells are produced in lymphoid organs and tissue from haematopoietic pluripotent cells. Therefore, haematopoietic activity is regarded as a major factor in fish immunity.
Since the production of these defence cells is regulated by cytokines that bind the receptors present on the surface of these pluripotent stem cells (Zapata et al. 2006), cytokine production is also a key component in fish immunity. It would be overoptimistic to write about the function of Spirulina on each component of fish immunity, because no such data is available in literature. Thus, we reviewed the importance of Spirulina on fish health and immunity under the following four major headings.
Role of Spirulina in haematopoietic activity
Haematopoiesis is the process of producing blood cells such as white blood cells (WBCs), macrophages, natural killer cells, and granulocytes, which have a pivotal role in fish immunity. In fsh, the kidney and thymus are the primary haematopoietic organs. In blood cell genesis, multipotent progenitor cells which are descendants of haematopoietic stem cells (HSCs), are initially diferentiated to common lymphoid and myeloid progenitor cells (Fig. 2A). The lymphoid progenitor cells proliferate and diferentiate into T and B lymphocytes, which are associated with specifc immunity. In contrast, common myeloid progenitor cells take part in non-specifc immunity by producing platelets, neutrophils, eosinophils, monocytes, dendritic cells, as shown in Fig. 2A (Kulkeaw and Sugiyama 2012; Orkin and Zon 2008). The quantity of those blood cells is an essential indicator of fsh health status that is regulated by combination of extrinsic and intrinsic factors (Kulkeaw and Sugiyama 2012). Many works have been conducted to measure the haematopoietic activity induced by Spirulina on diferent fsh species like African catfsh (Promya and Chitmanat 2011), Caspian brown trout (Meshkat Roohani et al. 2020), Rohu fngerlings (Andrews et al. 2011), and Nile tilapia (Hassaan et al. 2021; Ibrahem et al. 2013; Mabrouk et al. 2022; Mahmoud et al. 2018; Ragap et al. 2012; Şahan 2015). Spirulina enhances the count of red blood cells (RBCs), their volume percentage in blood (haematocrit) and haemoglobin content as mentioned in Table 1 (Abdel-tawwab et al. 2021; Abdel-Tawwab and Ahmad 2009; Meshkat Roohani et al. 2020; Mohammadiazarm et al. 2021; Promya and Chitmanat 2011; Şahan 2015). Iron is a major component of haemoglobin and plays an active role in the maturation of RBC. Spirulina biomass is a rich source of iron (50 mg per 100 g biomass), which is an excellent dietary supplement for treating anaemia in humans (Paula da Silva et al. 2021). It is presumed that the iron in Spirulina helps in erythropoiesis in fsh, the process by which RBC is produced (Hamed et al. 2019; Yu et al. 2018). In addition, carotenoids and phycobilin pigments like phycoerythrin, phycocyanin, and allophycocyanin reduce the RBC haemolysis caused by peroxide radicals (Abdel-Tawwab and Ahmad 2009; Andrews et al. 2011; Kop and Durmaz 2008; Romay et al. 2003). These Spirulina pigments scavenge superoxide anions, produced due to oxidative stress in the RBC and other blood cells which are very destructive to biomolecules and plasma membranes (Andrews et al. 2011; Meshkat Roohani et al. 2020). WBC augmentation was reported in many fsh species fed with Spirulina diet similar to RBC (Mamun et al. 2023; Rahman et al. 2023; Şahan 2015; Yu et al. 2018). There are also increasing number of research evidences (Table 1) that showed higher lysozyme production and phagocytic activity in fsh after Spirulina enriched diet consumption and eventually it reduces the fsh mortality during bacterial infection (AbdelLatif et al. 2022c; Cao et al. 2018; El-Araby et al. 2022; Güroy et al. 2022; Ibrahem et al. 2013; Mamun et al. 2023; Mohamed et al. 2023; Ragap et al. 2012; Siddik et al. 2022). For instance, Adel and colleagues, 2016 showed that fsh feed made up of 10% Spirulina increased the antibacterial activity and protection from pathogens in the skin mucus of the great sturgeon against the bacterium, Streptococcus iniae (Adel et al. 2016a). In another feld trial experiment with juvenile olive founder, Paralichthys olivaceus, lysozyme activity was increased signifcantly with the dietary supplementation of 3.4% Spirulina (Kim et al. 2013a). Neutrophils, monocytes, and macrophages are involved in phagocytosis, in which invaded pathogens are ingested and eliminated by oxygen free radicals such as superoxide anions, hydrogen peroxide, and hydroxyl free radicals through respiratory burst activity (Kim et al. 2013b; Panigrahi et al. 2005; Taoka et al. 2006; Yu et al. 2018). The diets made up of Spirulina increase the phagocytic and natural killer cell activity in fsh (Adel et al. 2016a; Mahmoud et al. 2018; Watanuki et al. 2006). Spirulina also helps in mitigating the immunosuppressive efects on fsh health due to the presence of herbicides and insecticides. Herbicides and insecticides which are widely used in agriculture, suppress immune responses such as erythropoiesis, WBCs count, the expression of the lysozyme gene, phagocytic and antibacterial activity in fsh (Abdel-tawwab et al. 2021; Abdel-Latif et al. 2022a). The diets supplemented with Spirulina, most precisely iron and phycocyanin components, enhance erythrocytes production, phagocytosis, and expression of lysozyme in common carp and Nile tilapia, exposed to atrazine and imidacloprid contamination respectively (Abdel-tawwab et al. 2021; Khalil et al. 2017). In addition to blood cells, the Spirulina diet increases the serum total protein, albumin, and globulin in fsh (Raji et al. 2018; Yeganeh et al. 2015). These blood proteins are involved in the transport of diferent minerals and essential chemicals like calcium, magnesium, free fatty acids, thyroxine, bilirubin, cortisol, and oestrogen for regular physiological as well as immunological activities. Several studies demonstrated that Spirulina-fed fsh maintain a normal level of these serum proteins during illness or malnutrition (Abdel-Tawwab and Ahmad 2009; Alexander et al. 2011). Elevation in the ratio of albumin and globulin coincides with the decrease in production of immunoglobulin; -that’s why serum protein is also very essential for the immune response.
The specifc components of Spirulina that elicits hematopoietic activities and their haematopoietic pathway are not fully identifed in the fsh system. The more detailed work on the mammalian system infuenced fsh biologists to conclude that Spirulina components like polysaccharide, C-phycocyanin act as erythropoietin and stimulate the haematopoietic organs to produce red blood cells and immune cells (El et al. 2023; Hamed et al. 2019; Hayashi et al. 2006; Sacha et al. 2005). As phycocyanin possesses erythropoietin like (Epo) activity, it may bind erythropoietin receptor (EpoR) and block apoptotic gene expression, promote proliferation and diferentiation of haematopoietic progenitor cells through the Janus Kinase and Signal Transducer and Activator of Transcription 5 (JAK/STAT5) signalling pathway (Fig. 2B) (Hattangadi et al. 2011). Phycocyanin also activates macrophages, granulocytes, as well as heals tissue damage caused by infammation (Meshkat Roohani et al. 2020; Selmi et al. 2011). Most research speculated that Spirulina components such as phycocyanin, phycoerythrin, polysaccharide, iron, Vitamin B12, Vitamin A, Vitamin E are responsible for stimulating haematopoietic activity and strengthening the fsh health (Fig. 2C). Shedding light on the efect of Spirulina on the haematopoietic pathway and eliciting the mechanism could lead to better use of Spirulina in fsh immunity development.