1. The Interferon family
Interferons (IFN) were first discovered by Isaacs and Lindemann in 1957. The initial experiments were performed in chick chorioallantoic membranes in a nutrient fluid where the addition of influenza virus stimulated the production of a protein which interfered or prevented viral replication. In 1965, IFNγ was discovered as a viral inhibitory protein produced by lymphocytes in response to mitogen stimulation. Though initially named immune IFN, it was later renamed IFNγ. Subsequently, different types of IFN have been identified and categorized into three families: Types I–III [1]. The focus of this review will be on IFNγ, a Type II IFN. IFNγ is widely distributed, from puffer fish to humans and plays pivotal roles in host defense (reviewed in [2–4]).
The importance of IFNγ is reflected in patients lacking IFNγ or its receptors or its key signaling molecules. These patients display increased susceptibility to microbial infections, e.g. fatal dissemination of Bacillus CalmetteGuerin during infancy [5,6]. IFNγ has been used to treat several diseases and malignancies: chronic granulomatous disease (CGD) is an inherited disorder of leukocyte function caused by defects in nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, the enzyme complex responsible for phagocyte superoxide generation. Recurrent life-threatening bacterial and fungal infections, as well as abnormally exuberant inflammatory responses, are common in CGD. Infections are dramatically reduced by prophylaxis with antibiotics, antifungals, and IFNγ. In fact, the prolonged use of IFNγ in patients with CGD appears to be safe and shows persistent reduction in the frequency of serious infection and mortality [7]. Similarly, in severely immunosuppressed patients suffering from acute myelogenous leukemia, IFNγ is being used for treatment of invasive fungal infections. Also, in difficult-to-treat fungal infections, the addition of IFNγ appears to improve the outcome of the treatment regime [8]. These studies underscore the importance of IFNγ in host defense and its potential as a therapeutic agent for selected maladies.
2. IFNγ signaling
IFNγ is secreted by activated T cells, natural killer (NK) cells and macrophages. Mature IFNγ is a protein of 143 amino acids with a molecular weight of 20 kDa. IFNγ is an acid-labile and dimeric cytokine and each monomer consists of a core of six a-helices and an extended unfolded sequence in the C-terminal region [9]. The biologically active dimer is formed by anti-parallel inter-locking of two monomers [10]. The binding of IFNγ to its receptor activates the JAK-STAT pathway which modulates the transcriptional activation of several genes and mediates diverse biological responses (Fig. 1).
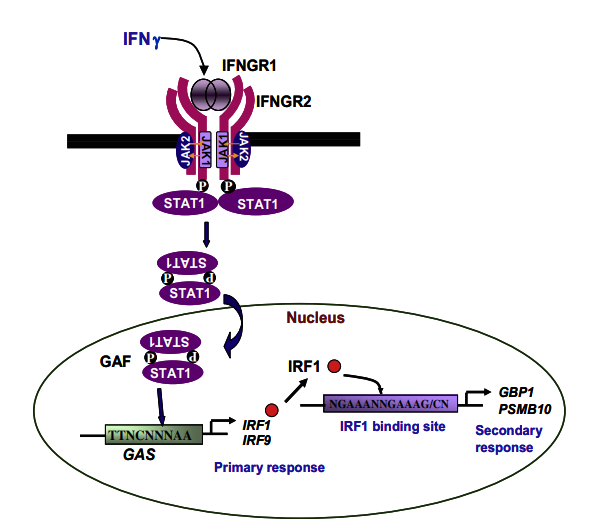
Fig. 1. The IFNγ signaling pathway. Binding of IFNγ results in receptor oligomerisation followed by trans-phosphorylation and activation of JAK1 and JAK2. Activated JAKs phosphorylate IFNGR1 resulting in docking of STAT1. Phosphorylated STAT1 homodimerizes to form Gamma-Activated Factor which binds to GAS located in the promoters of several primary response genes and increases transcription by recruiting several coactivators. Upon induction, the transcription factor IRF1 binds to ISRE and enhances transcription of several secondary response genes.
The IFNγ receptor consists of two subunits, IFNγ receptor (IFNGR)1 and IFNGR2, and each molecule interacts with a member of the JAK family, which are non-receptor protein tyrosine kinases. JAKs phosphorylate receptors and transcriptional coactivators known as STATs (reviewed in [11]).
IFNγ binds to its receptors with high affinity and in a speciesspecific manner, i.e. human IFNγ does not bind to the mouse IFNGR and vice versa. IFNGR1, the larger subunit, is required for ligand binding and its carboxy terminus is involved in binding to JAK1 and phosphorylated STAT1.
The smaller subunit, IFNGR2, is required for signaling and contains the JAK2 binding site. After engagement of the IFNGR with IFNγ, phosphorylation of the receptor generates a binding site for STAT1 via its Src homology 2 (SH2) domain. Phosphorylation of STAT1 on Tyr701 results in the formation of STAT1 homodimers, known as Gamma-Activated Factor, which translocate to the nucleus, bind to GAS and enhances transcriptional activation by recruiting several transcriptional coactivators. (Fig. 1).
For optimal activity, STAT1 needs to be also phosphorylated on Ser727 [12]. The importance of this phosphorylation is demonstrated in mutant mice expressing the Ser727Ala mutant of Stat1, which shows increased mortality upon infection with Listeria monocytogenes [13]. Importantly, Stat1/ mice do not signal via IFNab or IFNγ and are extremely susceptible to microbial infections [14].
IFNγ signaling has been studied for several years; however, accumulating evidence clearly demonstrates the roles of other pathways. Activation of phosphatidylinsositol-3-kinase (PI3K) by IFNγ seems to have important functional consequences in IFNγinducible transcriptional regulation. Inhibition of PI3K blocks the IFNγ-dependent phosphorylation of STAT1 on Ser727 and reduces STAT1 driven transcription. IFNγ activation of the PI3K pathway also leads to activation of Protein kinase C (PKC) ε , which activates the mitogen activated protein kinase (MAPK) pathway, and transcriptional activation of STAT1 [15].
IFN are also involved in transcriptional activation of PKC θ , which in turn activates MAPK kinase 4 [16]. In addition, PKC δ is activated by treatment of cells with IFNγ and has been shown to associate with STAT1 [17]. IFNγ activates various MAPK pathways leading to regulation of cell growth, differentiation, apoptosis, etc. In response to stress (e.g. UV or LPS), STAT1 is phosphorylated on Ser727 by p38 MAPK [18].
In bone marrow derived primary macrophages, IFNγ induces p38 MAPK and increases the expression of genes involved in chemotaxis and inflammation, e.g. CXCL10 (IP10), TNFa and NOS2 (iNOS). Although Extracellular signal-regulated kinases and c-Jun N-terminal kinases (JNK) are also activated by IFNγ, the extracellular signal-regulated kinases pathway has modest effects on pro-inflammatory gene expression whereas JNK regulates the expression of genes involved in antigen presentation, e.g. CIITA. Therefore, IFNγ activation of MAPK selectively modulates macrophage responses [19].
Further studies are required to fully comprehend IFNγ signaling and the cross talk that occurs across pathways. The activation of STAT1 by IFNγ is not continuous and is inhibited after some time, suggesting the presence of negative regulators of IFNc signaling. One of the best studied negative regulators of the JAK-STAT pathway are suppressor of cytokine signaling (SOCS) proteins which inhibit JAK activity [20,21]. In addition, the SH2 domain-containing tyrosine phosphatase (SHP2) has been implicated as a negative regulator of IFNγ signaling: Motheaten mice lack this enzyme and display high levels of Jak1 and Stat1 phosphorylation together with increased binding of Gamma-Activated factor to Gas [22]. Not surprisingly, Shp2/ cells are more sensitive to IFNγ [23] and, under conditions when SHP2 expression is increased, IFNγ signaling is reduced [24].
3. Modulation of gene expression by IFNγ
Cellular responses mediated by IFN are, primarily, due to modulation of gene expression. Therefore, identification and studying the roles of IFN-stimulated genes (ISG) during immune responses is an active area of investigation. IFNγ-modulated genes can be classified into primary or secondary responsive genes. Primary responsive genes are induced early due to the binding of Gamma-Activated Factor (STAT1 dimers) to GAS present in promoters of target genes, e.g. IRF1, CXCL9 (MIG1) and CXCL10 [2]. The secondary responsive genes are induced following the binding of IRF1 to Interferon stimulated response elements (ISRE) located in promoters of target genes (Fig. 1).
IRFs belong to a family of transcription factors consisting of IRF1, IRF2, and IRF8 (also known as IFN consensus sequence binding protein, ICSBP). Unlike IRF1, IRF8 does not bind directly to the ISRE but can activate transcription of ISRE containing genes as a heterodimer with IRF1 (reviewed in [25]). IFNγ-induced gene expression is modulated by different mediators and the cellular environment. For example, most IFNγ responsive genes are dependent on Stat1 although some genes, e.g. Ccl2 (Mcp1), Pim1, Fn1, etc. are Stat1-independent [26]. Breast cancer 1 (also known as BRCA1), the tumor suppressor, together with STAT1 differentially activates a subset of ISGs [27].
Also, IFNγ has been shown to activate a subset of genes, Cxcl10, Psmb8, Mx1, Ndr1, etc., in an Inhibitor of kappa B kinase-dependent manner [28]. In addition, reactive oxygen species (ROS) and reactive nitrogen intermediates (RNI) have been shown to modulate gene expression by IFNγ. In fact, IFNγ-modulated genes can be categorized into two distinct sets: oxidative and nitrosative stress-independent (Tap2, Psmb10) and oxidative and nitrosative stress-dependent (Nos2, Cybb (gp91 phox), Id2) [29].
These studies highlight the complex nature of IFNγ-induced gene expression. As GAS (TTNCNNNAA) and ISRE (NGAAANNGAAAG/CN) are important during IFNγ-mediated gene expression, an in silico analysis was performed to study the presence of these RE in the putative promoters of 228 genes that are involved in the immune response (Supplementary Table I) or randomly selected genes from the microarray data set that are not directly related to immunity (Supplementary Table II). For better comparison, the presence of RE that bind to two well known transcription factors, NFjB and AP1, were also studied. Sequences 1000 base pairs upstream to 50 bps downstream of the putative transcriptional start site were extracted from the ‘‘CSHL mammalian promoter database” (http://rulai.cshl.edu/cgi-bin/CSHLmpd2/promExtract.pl). Using a pattern search program ‘‘seqool” (http://www.biossc.de/seqool/ index.html), GAS, ISRE, NFKB-RE (GGGRNNYYCC) and AP1-RE (TGAG TCA) were identified in the putative promoters.
To enhance the stringency of analysis, only genes that contained RE for a transcription factor in both mouse and human orthologs were considered to be positive. The detailed procedure used for this analysis is described in the Supplementary protocols. As seen in Supplementary Fig. 1, a high percentage of immune related genes contained GAS (54.3%) and, in comparison, the presence of ISRE (4.3%), NFKB-RE (12.2%), and AP1-RE (4.8%) were low. Surprisingly, GAS was also present in higher number of genes that are not directly related to immunity. The Venn diagram shows the number of genes that contained GAS, either alone or in combination with another transcription factor-RE (Fig. 2A). Notably, immune genes showed greater association of GAS and an additional transcription factor RE. The functional relevance of the data was analyzed by studying the expression of GAS containing genes in microarray studies from three cells stimulated with IFNγ: NIH 3T3 cells [30], human fetal microglial cells [31] and mouse astrocytes [32].
As observed in Fig. 2B, the expression of 15–25% of GAS containing immune genes were up-regulated, which is significantly higher compared to genes not directly related to immunity. A list of selected GAS containing immune related genes that were regulated by IFNγ is displayed in Table 1. Interestingly, the percentage of immune genes that contained GAS and another transcription factor RE (ISRE or NFKB-RE or AP1-RE) were up-regulated higher as compared to immune genes containing GAS alone (Fig. 2C). This analysis demonstrates a strong correlation between immune function related genes with the presence of GAS and IFNγ-regulated gene expression. It is possible that the strategies used in this analysis may identify novel IFNγ-regulated genes that may play roles in the immune response. This information is further integrated with the existing knowledge on the roles of IFNγ in regulating gene expression and different biological responses.