INTRODUCTION
Viruses are the most abundant life form on earth, inhabiting nearly every ecosystem, including animals, plants, and bacteria. Research over the past decade has provided enormous insights into the mechanism by which viruses are detected by infected cells. It is clear that the innate immune system is equipped with multiple sensors that detect different molecular signatures of a viral infection.
Some sensors are expressed in specialized cell types, whereas others are virtually ubiquitous. The principle of innate virus recognition falls largely into two categories: recognition of pathogen-associated molecular patterns (PAMPs) via pattern recognition receptors (PRRs) and detection of pathogen-inflicted damage or stress. Viral PAMPs often carry distinct molecular or subcellular signatures not found in host cells, such as unique molecular features of the viral genome or viral replication intermediates.
On the other hand, stress or damage inflicted by viral infection is recognized through pathways that are shared with other stress-sensing pathways. The reader is referred to many excellent reviews on molecular descriptions of sensors, signaling pathways, and antiviral effectors elicited by innate viral recognition (2, 70, 92, 98). This review attempts to describe innate virus recognition from a virological perspective. I describe recent developments in our understanding of innate virus recognition by focusing on viral replication and invasion strategies, and highlight unexplored features of viral infections that might serve as signatures recognized by the innate immune system.
PATHWAYS ENGAGED FOLLOWING INNATE VIRUS RECOGNITION
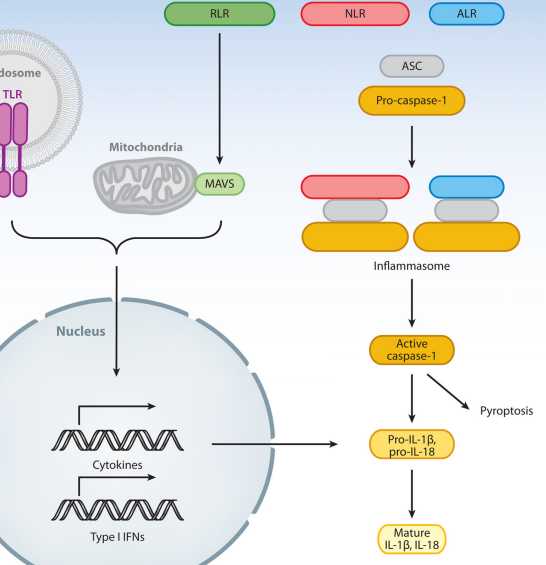
Innate sensors of viruses induce two distinct outcomes (Figure 1). The first outcome is that the engagement of PRRs induces signals, resulting in the transcriptional activation of cytokines and type I interferon (IFN) genes. Most cytokines are downstream of the transcription factor NF-κB, while the IFN genes are regulated by interferon regulatory factors 3 and 7 (IRF3 and IRF7) (39).
Nuclear Factor-kappaB(NF-κB)
The second outcome of PRR engagement is the activation of caspase-1 through the formation of inflammasomes. The inflammasomes enable proteolytic activation of caspase-1, which in turn can cleave multiple substrates including pro-interleukin (IL)-1β and pro-IL-18 (62).
The posttranslational modification (caspase-1 cleavage) of these cytokines is required for their extracellular release and activity (Figure 1). Both PRR-induced transcriptional and inflammasome pathways can also engage programmed cell death through apoptosis and pyroptosis, respectively, in an effort to prevent pathogen replication and spread. Here, we consider natural viral ligands for PRRs that engage these two types of biological outcomes.

Figure 1. Pathways engaged following activation of innate viral sensors. TLRs reside either on the cell surface or in the endosomes; the latter requires cleavage for signaling. RLRs are present in the cytosol. Upon engagement of TLRs and RLRs by viruses, the receptor transmits signals that lead to the transcriptional activation of hundreds of genes including cytokines and type I IFNs. NLR and ALR proteins are localized in the cytosol. Certain virus infection leads to the activation of these receptors to form inflammasome, a large multimeric complex consisting of a subset of NLR/ALR, ASC, and pro-caspase-1. Caspase-1 becomes activated and cleaves its substrates including pro-IL-1β and pro-IL-18 for extracellular release. Cross talks between these pathways and exceptions are discussed throughout this review. Abbreviations: TLR, Toll-like receptor; RLR, RIG-I-like receptor; ALR, AIM2-like receptor; ASC, apoptosis-associated speck-like protein containing a caspase recruitment domain; MAVS, mitochondria antiviral signaling protein; NLR, Nod-like receptor; IL, interleukin; IFN, interferon.
NUCLEIC ACID–BASED VIRAL RECOGNITION
There are an estimated 1031 viruses on earth (14). In 1971, David Baltimore proposed a classification of viruses based on the mechanism of mRNA production (7). According to the Baltimore classification, all viruses fall into one of seven groups depending on a combination of their genomes (DNA, RNA), strandedness (single or double), sense (sense or antisense), and mode of replication (Figure 2).
I utilize this classification throughout this review, as it is particularly useful for understanding distinct modes of innate viral recognition strategies. The virus genome and the viral strategy used to generate mRNA from its genome provide a suitable framework to classify host innate sensors.
The best characterized mode of innate viral recognition is the detection of viral nucleic acids. Nucleic acid–based recognition can sense either virion-associated viral genomes (replication independent) or replication products, including the whole genome, replication intermediates, or viral transcripts.
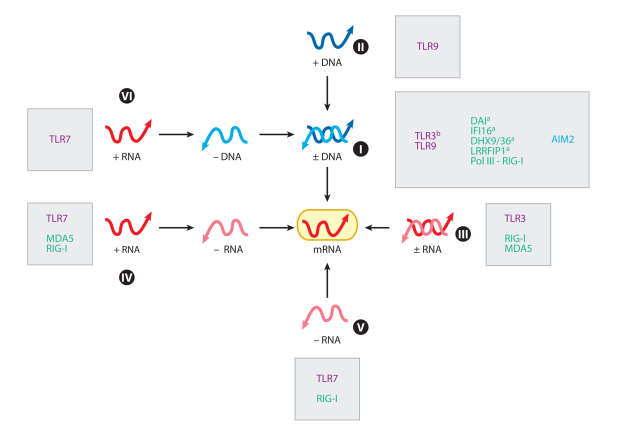
Figure 2. Innate sensors and Baltimore classification of viruses. All viruses fall into one of seven groups depending on a combination of their genomes (DNA, RNA), strandedness (single or double), sense (sense or antisense), and mode of replication. This classification enables innate sensors to be placed into functional categories. TLRs, RLRs, and other sensors that recognize respective groups of viruses are indicated. Superscript a denotes sensors that have been identified by genetic knockdown studies, and superscript b denotes sensors associated with virus-induced diseases in humans. Abbreviations: TLR, Toll-like receptor; RLR, RIGI-like receptor; mRNA, messenger RNA; Pol, polymerase; MDA5, melanoma differentiation-associated gene 5; RIG-I, retinoic-acid-inducible gene I; DAI, DNAdependent activator of interferon-regulatory factors; IFI, interferon-inducible protein; DHX, DEAH box protein; LRRFIP, leucine-rich repeat flightless-interacting protein.
Endosomal Recognition: Virion-Associated Viral Genomes and Viral Replication Intermediates
Toll-like receptors (TLRs) are innate sensors that detect PAMPs from a variety of pathogens (2). Many TLRs are expressed on the cell surface, but some are expressed in the endosomes, dedicated to recognizing viral genomes associated with virions (Figure 1). Upon endocytosis of viruses, endosomal TLRs sense viral genomes presumably after the envelopes and capsids are uncoated by the degradative enzymes therein, and trigger cytokine and type I IFN transcription.
envelope 외피

TLR7 and TLR9 recruit MyD88 and IRF7 to stimulate cytokine and type I IFN genes from the endosome. Signaling downstream of TLR7 and TLR9 is studied most extensively in a specialized cell type, plasmacytoid dendritic cells (pDCs), which use these receptors exclusively to recognize a wide array of viruses and produce copious amounts of type I IFNs (31).
These TLRs require proteolytic processing for signaling (26, 68). TLR9 recognizes double-stranded DNA (dsDNA) viral genomes of Group I viruses in the endosome (Figures 2 and 3). Recognition via TLR9 does not require viral replication nor sequence-specific motifs (33). Group II viruses contain single-stranded DNA (ssDNA) genomes, and a member of this group of viruses (adeno-associated virus) stimulates TLR9 (109). TLR7 senses ssRNA viral genomes of Group IV, V, and VI viruses in the endosome.
proteolytic 단백질 가수분해


Figure 3. Known and putative viral PAMPs. Innate sensors can detect viral genomes in the endosomes ( purple boxes) or in the cytosol inside infected cells ( yellow boxes). Green letters denote cytosolic sensors, purple letters denote endosomal sensors, and blue letters denote antiviral effector ISGs. Host counterparts, where appropriate, are depicted at the bottom. Viral signatures predicted to serve as PAMPs are indicated by the pink boxes. These include sfRNA (Group IV), cap 0 structure of Sindbis virus mRNA, ssDNA in the nucleus (Group II), circular DNA (Group I, II), ssDNA and dsDNA in the cytosol (Group VI), and leader RNA (Group V). Abbreviations: PAMP, pathogen-associated molecular pattern; ISG, interferon-stimulated gene; sfRNA, subgenomic Flavivirus RNA; ssDNA, single-stranded DNA; dsDNA, double-stranded DNA; PKR, double-stranded RNA-activated protein kinase; OAS, 2′,5′-oligoadenylate synthase; RIG-I, retinoic-acid-inducible gene I; IFIT, interferoninduced tetratricopeptide repeat protein; MDA5, melanoma differentiation-associated protein 5; TREX1, three prime repair exonuclease 1; TLR, Toll-like receptor; DI, defective interfering; EBV, Epstein-Barr virus; EBER, EBV-encoded RNA; DHX, DEAH box protein; ppp, triphosphate; pA, poly(A) tail; Vpg, viral protein genome-linked; mRNA, messenger RNA; DAI, DNA-dependent activator of interferon-regulatory factors; AIM2, absent in melanoma 2; KSHV, Kaposi’s sarcoma-associated herpesvirus; IRES, internal ribosomal entry site; tRNA, transfer RNA.
In humans, TLR8 is expressed by myeloid dendritic cells (DCs) and is similarly capable of recognizing ssRNA in the endosome (36). Uridine and ribose, the defining signatures of RNA, are both necessary and sufficient for TLR7 stimulation (23). Whereas influenza virus (6, 22) and retrovirus genomes (12, 46) are recognized via TLR7 in a replicationindependent manner, other ssRNA viruses, such as vesicular stomatitis virus (VSV) and paramyxoviruses, require replication (55) and autophagy for recognition by TLR7 (55, 61).
Why some RNA viruses require replication and autophagy for TLR7 recognition while others do not is unclear. Autophagy-dependent recognition may be required for viruses that fuse at the plasma membrane or escape endosomes before they progress to the late maturation stage needed for TLR signaling (10). In this case, cytosolic viral RNA is delivered to the TLR-containing endolysosomes via autophagy.
Another intriguing correlation is that the viruses that are recognized independently of autophagy replicate in the nucleus, whereas those dependent on autophagy for recognition replicate in the cytosol.
TLR3 was originally identified as a sensor of dsRNA viruses, as TLR3-deficient splenocytes failed to upregulate CD69 upon stimulation with an isolated reovirus genome (3). However, TLR3 is not required for innate or adaptive immune responses against lymphocytic choriomeningitis virus (LCMV), VSV, murine cytomegalovirus (MCMV), and reovirus (24).
Instead, TLR3 may be important in detecting virally infected cells when they are phagocytosed by DCs for cross-priming (89). In humans, genetic deficiencies in TLR3 and its signaling pathway have been associated with herpes simplex encephalitis (107), indicating that TLR3 may play a key role in protecting the central nervous system against herpes simplex virus 1 (HSV-1) infection (Figure 2). Recent data indicate that TLR3- deficient mice succumb to central nervous system infection upon vaginal HSV-2 challenge due to a lack of type I IFN production by astrocytes (80), revealing the importance of innate recognition of HSV by TLR3 in the brain.
Cytosolic Recognition
Several classes of sensors detect viral infection in the cytosol. Cytosolic sensors can be divided in terms of structurally related family members:
RIG-I-like receptors (RLRs), which are sensors of RNA; AIM2-like receptors (ALRs), which are sensors of DNA; and nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs), which are sensors of viral PAMPs and virus-inflicted cellular stress (discussed in detail below) (Figure 1).
In general, RLRs signal via an adaptor molecule called the mitochondria antiviral signaling protein (MAVS) (90) [also known as IPS-1 (48), Cardif (64), or VISA (102)], which is found on the mitochondrial membrane (90). AIM2 (absent in melanoma 2) and NLRs form the inflammasome complex via an adaptor protein ASC (apoptosis-associated speck-like protein containing a caspase recruitment domain) within the cytosol.
Engagement of RLRs results in MAVS-dependent transcription of cytokines and type I IFNs; engagement of NLRs results in inflammasome activation (Figure 1), with some exceptions as described below. ALR stimulation results in either cytokine/IFN induction or inflammasome activation.
Innate recognition of replicated viral genomes in the cytosol—Retinoic-acidinducible gene I (RIG-I) recognizes 5′-ppp RNA upon infection with Group V viruses (41, 71) (Figure 2). Recognition through RIG-I requires replication, indicating that virionassociated genomes are not sufficient to serve as the viral ligand. A recent study showed that the majority of RIG-I-stimulating activity is associated with whole viral genomic RNA (vRNA) that is generated upon replication (Figure 3), but not with the complementary positive-strand RNA (cRNA) (79). Preferential recognition of vRNA to cRNA may be due to their ratio in infected cells. This study demonstrated that nongenomic viral transcripts, short replication intermediates, and cleaved self-RNA do not contribute substantially to IFN induction in cells infected with these negative-strand RNA viruses (79). However, RIG-I may also recognize partial replication intermediates containing 5′ ppp as described below.
Innate recognition of viral replication intermediates in the cytosol—In addition to the whole genome, viral replication intermediates serve as PAMPs for cytosolic viral sensors. Unique RNA and DNA intermediates generated in the course of virus infection are recognized by different groups of innate sensors and effector molecules.
RNA sensors: Evidence indicates that virus infection generates distinct species of RNA that are recognized by host sensors.
- RIG-I: In an effort to obtain unbiased information on RIG-I ligand during viral infection, Baum et al. (11) performed deep sequencing of RIG-I-bound RNA from cells infected with the Sendai virus (Cantell strain) and influenza (strain delta NS1). This study revealed that RIG-I specifically bound the genome of the defective interfering (DI) particle and did not bind the full-length virus genome or any other viral RNAs in Sendai virus–infected cells (Figure 3). In influenza-infected cells RIG-I preferentially bound shorter genomic segments as well as subgenomic DI particles. Other reports demonstrated that RIG-I detects negativestrand RNA viruses that contain blunt, short, double-stranded 5′-triphosphate RNA (88) in the panhandle region of their single-stranded genome (87). These reports collectively indicate that a major viral RIG-I ligand is the short dsRNA with a 5′-ppp end, likely a DI particle (Figure 3). In addition to Group V viruses, RIG-I is the primary sensor for hepatitis C virus (HCV) (Group IV) (30). Of note, the HCV genome has 5′ ppp (Figure 3). When various regions of the HCV genome were tested, the polyuridine motif of the 3′-untranslated region and its replication intermediate were identified to be the PAMP substrate of RIG-I (84). Flaviviruses (genus Flavivirus), but not other genera of the Flaviviridae family (such as HCV), produce a unique, small, noncoding RNA (~0.5 kb) derived from the 3′-untranslated region of the genomic RNA, which is required for their cytopathicity and pathogenicity (73) as well as replication (27). This subgenomic Flavivirus RNA (sfRNA) is a product of incomplete degradation of vRNA by cellular ribonucleases (73) (Figure 3). It is tempting to speculate that sfRNA may bind to an RNA sensor, such as RIG-I, to inhibit innate signaling.
- MDA5: MDA5 belongs to the RLR family. Unlike RIG-I, MDA5 does not recognize 5′-ppp RNA. Instead, replication intermediates generated upon infection with Group IV viruses are recognized. Some flaviviruses (Group IV) are recognized by both RIG-I and MDA5 (dengue, West Nile Virus), where others are recognized only by RIG-I ( Japanese encephalitis virus, HCV) (57). Reovirus (Group III) infection is also recognized by both RIG-I and MDA5 (57). The precise target of MDA5 is still unclear. The DI particle generated in Sendai virus–infected cells can activate MDA5 and induce IRF3-dependent genes (Figure 3). DI particles can overcome viral immune evasion via the Sendai virus V protein (106). Another study reported that highly structured RNA in infected cells is recognized by MDA5 (72). Interestingly, Sendai virus infection, which is known to trigger RIG-I but not MDA5 in vitro (47), revealed the importance of MDA5 in antiviral defense in vivo (32). Type I IFNs and cytokine responses were intact in MDA5-deficient mice until day 5 postinfection, suggesting that MDA5 may induce an IFN-independent antiviral program that is not entirely countered by the Sendai virus V protein.
- Other sensors: NOD2 is a member of the NLR. A report (82) indicated that NOD2 binds to MAVS and induces IRF3 activation upon Group V ssRNA viral infections (respiratory syncytial virus, VSV, parainfluenza virus) but not following vaccinia virus (VV) (Group I, DNA) infection. Ultra-violet treatment of viruses diminished IRF3-dependent type I IFN response, suggesting that NOD2 is stimulated by RNA generated after Group V viral infection (82). In another report, in human peripheral blood mononuclear cells or mouse bone-marrow-derived DCs, VSV was shown to activate the RIG-I/ASC/caspase-1 inflammasome, independent of Nod-like receptor protein 3 (NLRP3) (75). In this case, RIGI senses viral RNA and activates caspase-1 instead of activating type I IFN synthesis downstream of MAVS. This study showed that synthetic 5′-ppp RNA also triggers inflammasomes in a RIG-I-dependent manner. It remains unclear under what circumstances RIG-I induces cytokine/IFN transcription via MAVS versus inflammasome activation via ASC.
RNA-sensing executors : In addition to RNA sensors that trigger the synthesis of IFNs and cytokines, signatures of viral RNA are sensed by the effector molecules that carry out antiviral functions. These effector molecules are themselves IFN induced and require the presence of unique RNA structures synthesized during viral infection for their activity. Both double-stranded RNA-activated protein kinase (PKR) and 2′,5′-oligoadenylate synthase bind to dsRNA to become active enzymes (83).
Most viral infections result in dsRNA synthesis during their replication cycle (Figure 3). A recent study has identified that interferon-stimulated genes (ISGs), IFIT1, IFIT2, and IFIT3 (interferon-induced tetratricopeptide repeat protein 1/2/3), bind 5′-ppp RNA in order to restrict replication of Group V viruses, including Rift Valley fever virus, VSV, and influenza A virus (69). Thus, in addition to RIG-I, IFIT proteins utilize 5′-ppp RNA to execute their viral restriction effector function. In addition, as discussed below, IFIT1 and IFIT2 also sense viral mRNAs that lack 2′ O-methylation (20, 111) in order to restrict viral replication.
DNA sensors: Multiple sensors recognize DNA in the cytosol from various sources including DNA from viruses, bacteria, and apoptotic cells. Synthetic B-form DNA (poly dA:dT) and IFN stimulatory DNA (ISD) have been used to probe distinct pathways of cytosolic DNA recognition.
ISDs are dsDNA that contain oligonucleotides of at least 25 base pairs, which in a sequence-independent manner trigger the stimulation of type I IFNs downstream of TBK1 and IRF3 but not MAVS (93). Interestingly, ISD does not engage NFκB or MAPK pathways, thus activating only IRF3-induced pathways. The ISD recognition pathway exists only in primary cells and is lost from transformed cells (93). The search for DNA sensors of B-form DNA or ISD leading to IFN production is ongoing. DNAdependent activator of IRFs (DAI) (also known as DLM-1/ZBP1) was identified as a candidate intracellular DNA-sensing molecule (96). In vivo, DAI deficiency can be compensated for by other DNA-sensing receptors (44).
- IFI16/p204: IFI16 (interferon-inducible protein 16) and its mouse ortholog, p204, are members of the PYHIN (pyrin and HIN domain-containing protein) protein family, which contains a pyrin domain and two DNA-binding HIN (hemopoietic expression, interferoninducibility, nuclear localization) domains. Stimulation of IFI16 by HSV-1 infection triggers NF-κB and IRF3 activation in bone-marrow-derived macrophages (99). IFI16 is recruited to synthetic ds-DNA (60- or 70-mer) and STING (stimulator of interferon genes) in the cytosolic compartment. Requirement for IFI16/p204 for inhibiting viral replication appears to be restricted to MCMV and human cytomegalovirus (HCMV), as expression of p204 mutants had no effect on the replication of HSV-1, ectromelia virus, or VSV. In contrast to the role of IFI16 in IFN induction, IFI16 forms ASC-dependent inflammasomes after Kaposi’s sarcoma-associated herpesvirus infection in primary human endothelial cells (50), presumably upon recognition of nuclear viral DNA. The mechanism that determines whether IFI16 induces IFN or forms the inflammasome complex upon DNA viral infection is unknown.
- AIM2 : AIM2 is an IFN-inducible protein that contains the N-terminal pyrin domain and the C-terminal HIN-200 domain. The HIN-200 domain binds dsDNA in the cytosol. AIM2 recognizes certain dsDNA viruses (MCMV and VV, but not HSV-1) upon infection in primed macrophages (28, 40) (Table 1). AIM2 is also triggered by synthetic dsDNA poly dA:dT in phorbol myristate acetate–differentiated THP-1 cells (15) or in mouse bonemarrow-derived macrophages (81). In macrophages primed with TLR ligands, cytosolic : AIM2 is an IFN-inducible protein that contains the N-terminal pyrin domain and the C-terminal HIN-200 domain. The HIN-200 domain binds dsDNA in the cytosol. AIM2 recognizes certain dsDNA viruses (MCMV and VV, but not HSV-1) upon infection in primed macrophages (28, 40) (Table 1). AIM2 is also triggered by synthetic dsDNA poly dA:dT in phorbol myristate acetate–differentiated THP-1 cells (15) or in mouse bonemarrow-derived macrophages (81). In macrophages primed with TLR ligands, cytosolic dsDNA binds to AIM2, leading to formation of an inflammasome consisting of AIM2/ASC/ pro-caspase-1. Although not formally tested, pyroptosis following AIM2 inflammasome activation likely has an antiviral role by eliminating infected cells.
- DHX9 AND DHX36: DHX9 and DHX36 are aspartate-glutamate-any amino acid-aspartate/ histidine (DExD/H)-box helicase (DHX) proteins that localize in the cytosol. In pDCs, DHX9 and DHX36 bind to synthetic oligodeoxynucleotides, CpG-A and CpG-B, respectively, and induce MyD88-dependent, TLR9-independent IFN production (51). The two forms of CpG-motif-containing oligodeoxynucleotides, CpG-A and CpG-B, have distinct molecular signatures and immunological phenotype in pDCs (49). Knocking down DHX9 or DHX36 reduces the cytokine responses of pDCs to HSV-1 but has no effect on the cytokine responses to influenza virus. Both DHX9 and DHX36 are localized within the cytosol and both are directly bound to the TIR domain of MyD88 via their helicaseassociated domain 2 and DUF domains. These molecules provide pDCs with a TLRindependent cytosolic DNA recognition mechanism consistent with residual cytokine secretion in TLR9-deficient pDCs infected with certain DNA viruses [HSV-1 and MCMV (37, 38) but not HSV-2 (59)] (Figure 3). Whether these molecules have a role in viral detection in non-pDC cell types is unknown.
- LRRFIP1 : An siRNA screening for molecules required for IFN-β production following Listeria monocytogenes infection identified LRRFIP1. LRRFIP1 binds to both B-form dsDNA and Z-form dsDNA (poly dG:dC), and it enhances the expression of IFN-β. Of note, LRRFIP1 enhances IFN-β production by both dsRNA (poly I:C) and dsDNA. LRRFIP1 promotes the activation of β-catenin, which increases IFN-β expression by binding to the Cterminal domain of the transcription factor IRF3 and recruiting the acetyltransferase p300 to the IFN-β enhanceosome via IRF3. Therefore, LRRFIP1 and its downstream partner βcatenin constitute a coactivator pathway for IRF3-mediated production of type I IFN (105).
DNA choppers : Presence of DNA in the cytosol is a hallmark of viral infection and can trigger a direct antiviral response. TREX1 (three prime repair exonuclease 1), a cytosolic exonuclease, is a negative regulator of the ISD pathway by inhibiting excess accumulation of DNA products from endogenous retroelements (91). This DNA exonuclease activity is important to prevent the autoimmune disease Aicardi-Goutieres syndrome in humans. However, TREX1 is also involved in degrading nonproductive reverse transcriptase products generated during HIV-1 infection, enabling HIV-1 to remain undetected by the putative DNA sensor (104) (Figure 3).
Innate recognition of DNA viral transcripts by host RNA polymerase III—In 2009, two independent studies (1, 18) demonstrated that RIG-I recognizes DNA viral infections. In infected cells, host RNA polymerase III (Pol III) transcribes a certain region of the DNA virus genome to produce 5′-ppp RNA, which becomes a target of recognition by RIG-I.
Pol III-dependent generation of RIG-I ligands can be mimicked by introducing poly dA:dT into the cytosol of cells by transfection. RNA Pol III normally transcribes cellular pre-tRNA, 5S rRNA, and U6 snRNA, all of which have conserved promoter sequences (Figure 3). Therefore, Pol III transcription is highly regulated and is sequence specific. What is the natural physiological viral ligand recognized by the Pol III–RIG-I pathway? Certain classes of viruses utilize Pol III to transcribe small, noncoding RNAs to counter host innate defense mechanisms, specifically PKR (Figure 4).
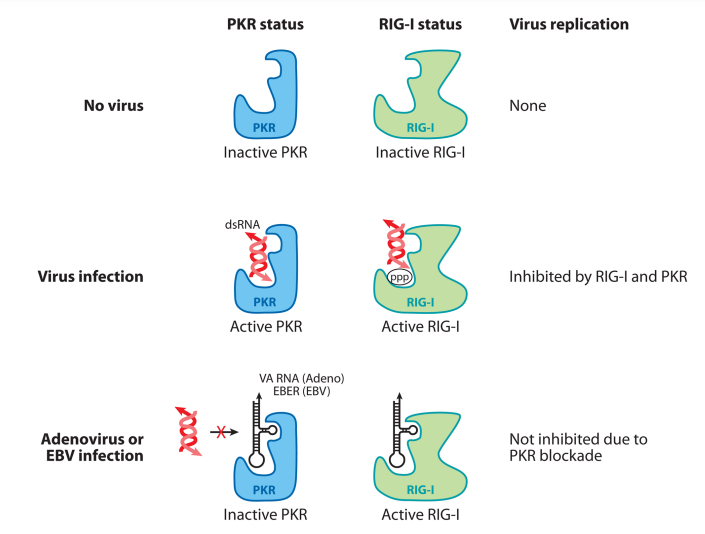
Figure 4. Pol III viral transcripts activate RIG-I but inhibit PKR. Pol III transcripts generated during adenoviral infection (VA RNA) and EBV infection (EBER) are small, noncoding RNAs that bind to and block PKR activation. In the absence of viral infection, neither PKR nor RIG-I is activated. In cells infected with a virus (excluding adenovirus and EBV), dsRNA structure generated in the cytosol triggers the activation of PKR and 5′-ppp RNA triggers RIG-I activation, resulting in an antiviral state. In cells infected with adenovirus or EBV, noncoding RNA Pol III transcripts bind to RIG-I and stimulate IFN synthesis. However, Pol III transcripts bind to PKR and block its activity by disabling binding of stimulatory dsRNA. Abbreviations: Pol, polymerase; VA RNA, viral associated RNA; EBV, Epstein-Barr virus; EBER, EBV-encoded RNA; RIG-I, retinoic-acid-inducible gene 1; PKR, double-stranded RNA-activated protein kinase; dsRNA, double-stranded RNA; IFN, interferon.
These viruses include adenovirus and Epstein-Barr virus (EBV). Therefore, the Pol III–RIG-I pathway of viral recognition represents a counter-countermeasure by the host to guarantee type I IFN production even in the face of viral evasion. However, as described below, the virus wins in the end by ensuring that the Pol III transcripts block the pathway downstream of type I IFN signaling. Adenovirus uses Pol III to generate small, noncoding viral associated RNAs (VA RNAs). VA RNAs are small, highly structurally conserved noncoding RNAs (~160 nucleotides in length) synthesized at high levels (108 copies per cell) during adenovirus replication (63). Adenovirus lacking VA RNAs induces minimal IFNs or cytokines in infected cells, indicating that VA RNAs are the primary target of recognition (103). VA RNAs are likely recognized by both RIG-I and MDA5, because mouse embryonic fibroblasts deficient in either of these molecules still respond robustly to adenovirus infection, whereas MAVSdeficient mouse embryonic fibroblasts are incapable of inducing IFN (103). VA RNAs are synthesized by the virus to counteract two host cell defense mechanisms: the PKR (52, 66, 100) and the Dicer/RNA-induced silencing complex (5) (Figure 4). VA RNAs bind to the dsRNA-binding domain of PKR and inactivate it. Similarly, VA RNAs bind to Dicer and act as a competitive inhibitor. In adenovirus mutants lacking one of the VA RNAs, PKR and Dicer are activated and viral replication is severely attenuated (52). Thus, even though RLRs can recognize VA RNAs to induce IFNs, adenovirus circumvents this by enabling VA RNAs to inactivate the downstream antiviral effectors.
EBV-encoded RNAs (EBERs) are structurally similar to the adenovirus VA RNAs, which are similarly small (~160–170 nucleotides) untranslated RNAs transcribed by Pol III. EBERs are expressed by EBV-infected cells during latency and are associated with resistance to apoptosis in Burkitt’s lymphoma. EBERs are recognized by RIG-I (1, 85). Like VA RNAs, EBERs also bind PKR, inhibit its phosphorylation, and thereby prevent type-I IFN-mediated apoptosis in infected cells. Interestingly, unlike the ISD pathway, the Pol III– RIG-I pathway is preserved in transformed cells (18), enabling EBV-transformed B cells to stimulate such a pathway. However, even though EBERs induce type I IFNs, as in the case of VA RNA, EBERs provide EBV a replicative advantage by blocking PKR and enabling translation of viral proteins (Figure 4).
Innate recognition of viral mRNA cap structures—All eukaryotic mRNA are modified at the 5′ end with a highly conserved cap structure shortly after the start of transcription. m7GpppN (referred to as cap 0) is further modified by cap-specific 2′-O RNA methyltransferases in the nucleus and cytoplasm that add a methyl group to ribose 2′- hydroxyl positions of the first and second nucleotides, giving rise to m7GpppNm (cap 1) and m7GpppNmNm (cap 2) structures, respectively. Viruses are equipped with various mechanisms to add 5′ cap to mimic the host mRNA. For example, 2′ O-methyl transferases are encoded by coronaviruses, VV, and flaviviruses to disguise their mRNA as “self.” The absence of 2′ O-methyl group is recognized by MDA5 (111), IFIT1, and IFIT2 (20, 111). Sindbis virus, an alphavirus, generates cap 0 structure, which is rarely found in mammalian mRNA in the cytosol (35). The 5′-terminal nucleotide of the Sindbis virus is modified such that the sequence is m7GpppApUpGp. This cap structure lacks a 2′ O-methyl group on both the first and the second nucleotides and represents a likely target of innate recognition by either PRRs (e.g., MDA5) or ISGs (e.g., IFIT1). To this end, the Sindbis virus uses its macromolecular host shutoff mechanism to ensure that the viral infection does not trigger IFN synthesis mediated by MDA5 (16). Whether this virus also blocks the functions of IFIT proteins would be interesting to explore.