I. INTRODUCTION
The immune system is dependent for its function on a complex set of regulatory interactions among many types of cells, including lymphocytes, monocytes, other types of leukocytes, and nonhemopoietic cells. These interactions are mediated both by cell-to-cell contact and by a variety of soluble factors, most notably a group of small regulatory proteins collectively known as cytokines.
Cytokines are produced by and act on both hemopoietic and nonhemopoietic cells, playing a key role in many physiologic processes, including inflammation, immunity, and hemopoiesis.2–5
The regulatory effects of cytokines on their target cells are mediated by membrane cytokine receptors, which after binding of their specific ligand (cytokine), initiate the activation of a number of enzymes and transcriptional factors, ultimately resulting in alterations to the gene expression and phenotypic characteristics of the cell.6–8
In the last few years, many laboratories have contributed to the elucidation of the structure and function of cytokine receptors, including their signaling mechanisms and association with defined families of protein kinases and transcriptional factors.8–10 Many studies have also demonstrated the existence of truncated, soluble forms of many of the different cytokine receptors.
The truncated receptors arise from the proteolytic cleavage of the membrane-bound receptors and/or from translation of alternatively spliced mRNAs different from those encoding the full-length membrane forms. These proteins are released from the cells and appear as soluble cytokine receptors (sCR) in biological fluids or tissue culture supernatants.11 From a functional standpoint, these sCR have the ability to influence the extracellular levels of their cytokine in vivo, and in many cases can act as competitive inhibitors of its interaction with the membrane cytokine receptors.
These properties, together with their commonplace existence, have led to the idea that the generation of sCR constitutes a natural physiological mechanism for regulating the activity of cytokines.11,12 In fact, ample evidence now suggests that the synthesis of many types of sCR is tied with that of their own ligands.13,14 The scientific interest in sCR has probably had just as much to do with their potential clinical applications as with the study of their basic immunoregulatory functions. The ability of sCR to specifically alter the biologic activity of their cytokines has made them very attractive as novel immunotherapeutic agents, particularly for clinical conditions in which cytokines play prominent pathophysiological roles, such as in inflammatory or autoimmune diseases.
In fact, the therapeutic potential of several sCR has already been studied in animal models and preclinical studies in humans. Furthermore, it has become increasingly apparent in the last few years that the levels of different sCR in biological fluids correlate with immune function and/or activation in many pathologic conditions and could be potentially used as helpful “markers” in the diagnosis, management, and/or prognosis of human diseases.13–15 Thus, the clinical impact of sCR extends to both experimental therapeutics and the clinical laboratory.
Despite our present knowledge, the roles of sCR in the regulation of immune responses, both in health and disease, have not yet been clearly established. Full development of the therapeutic potential of sCR will depend on our understanding of their basic properties, their effects on cytokine physiology, and their involvement in pathologic processes. Thus, a combination of basic and clinical studies will be paramount in order to achieve this goal. The purpose of this review is to discuss, in general terms, the mechanisms responsible for the regulation of cytokine activity in vivo, with particular emphasis on sCR, their biology, and potential clinical applications. Later on, more specific information on different sCR is presented, including their basic characteristics, associations with disease, and potential therapeutic applications.
II. CYTOKINES AND THEIR FUNCTIONS
The term “cytokine” applies to small regulatory proteins produced by many types of cells of both hemopoietic and nonhemopoietic lineages. This group of molecules encompasses those regulatory proteins secreted by T and B lymphocytes (lymphokines), monocytic cells (monokines), interferons, and hemopoietic colony stimulating factors (CSF).
Despite the fact that cytokines are a diverse group of proteins, they share a number of functional characteristics, including the ability to act on many different types of cells (pleiotropy) and to mimic other cytokines in their activity (redundancy).16
Because of the multiplicity of their functions and cellular origins, cytokines have not been easy to classify. Recently, however, families of cytokines have been recognized based on common genetic, structural, and signaling attributes.5 Nonetheless, because the main purpose of this work is to discuss the role of sCR receptors on the regulation of cytokine activity, a more “functional” classification of cytokines and their receptors has been preferred in this review. Cytokines have been grouped into four broad functional groups based on their major activities.4
These groups are (1) mediators of inflammation and nonspecific immunity; (2) regulators of lymphocyte function; (3) activators of nonspecific inflammatory cells; and (4) stimulators of immature leukocyte growth and differentiation.
A more detailed discussion of the different cytokines and their biologic activities is beyond the scope of this work, and readers are referred to several excellent reviews.16–22 Table 1 summarizes information on some of the cytokines considered as the major representatives of each functional group.

A. Mediators of Inflammation and Nonspecific Immunity
These cytokines are normally produced by mononuclear phagocytes in response to infectious or inflammatory agents, but can also be secreted by many other types of cells. Collectively, this group of cytokines is responsible for mediating inflammation and are also known as “proinflammatory cytokines.” Among the most important cytokines in this group are tumor necrosis factor-α (TNFα), interleukin-1 (IL-1), interleukin-6 (IL-6), and the chemokines.
These cytokines are responsible for both the local (e.g., increased vascular permeability, leukocyte infiltration, leukocyte activation), and systemic manifestations of inflammation (e.g., fever, acute phase response, leukocytosis, metabolic changes) and natural immunity against bacteria.18,20,22 Significantly, these cytokines, especially TNFα, also mediate the very serious and potentially fatal effects associated with systemic inflammatory responses (sepsis).20 Because of this reason, antagonists of TNFα and other proinflammatory cytokines might be particularly useful in the treatment of septic shock and chronic inflammatory diseases.15
B. Regulators of Lymphocyte Function
Most of the cytokines in this group are mainly produced by antigenactivated T lymphocytes, and their main function is to regulate the activation, proliferation, and differentiation of T and B lymphocytes. Thus, these cytokines play a central role in the development of both specific cellular and humoral immune responses.
Among these are (1) interleukin-2 (IL-2), the major T cell growth factor and important stimulator of human B cells and NK cells and (2) interleukin-4 (IL-4), a cytokine involved in B cell activation and the production of IgE, the principal mediator of immediate hypersensitivity (allergic) reactions.17,19 Because of their potential interference with the production of IgE antibodies by B cells, antagonists of IL-4 could be especially effective as anti-allergic agents.13
C. Activators of Nonspecific Inflammatory Cells
The cytokines in this group are also mainly derived from antigenactivated T lymphocytes, but their main function is the activation of nonspecific effector cells, such as monocytes, NK cells, eosinophils, and other leukocytes. Among the main cytokines in this group are (1) interferon-γ (IFNγ), a cytokine that as its name implies has antiviral activity, but is also the major macrophage-activating factor;21,23 (2) TNFβ or lymphotoxin, a cytokine related to TNFα; 20 (3) interleukin-12 (IL-12), an activator of NK cells and differentiation of naive CD4+ T cells into the Th1 subset;24 and (4) interleukin-5 (IL-5), whose main action is the enhancement of the growth and differentiation of eosinophils.25
D. Stimulators of Hemopoietic Growth and Differentiation
These are mononuclear cell and lymphocyte-derived cytokines whose main function is to stimulate the growth and differentiation of bone marrow progenitor cells. These cytokines vary in their specificity for different cell lineages (e.g., monocytic, granulocytic, erythrocytic).
Among the most important cytokines in this group are (1) interleukin-3 (IL-3), a multilineage stimulating factor; (2) granulocyte-macrophage-colony stimulating factor (GM-CSF); (3) monocyte-colony stimulating factor (M-CSF); (4) granulocyte-colony stimulating factor (G-CSF); and (5) interleukin-7 (IL-7), a cytokine that stimulates lymphoid progenitors.
III. REGULATION OF CYTOKINE ACTIVITY
The activity of cytokines is extremely complex. Most cytokines can be secreted by many different cell types in response to multiple types of stimuli.
In addition, cytokines are very potent mediators, acting at concentrations well below 100 pM and requiring occupancy of only a fraction of the membrane cytokine receptors on a cell (as low as 10%) in order to exert their biologic effects.
Moreover, some cytokines act to induce the synthesis of other cytokines, leading to cascades and networks of interacting cytokines, resulting in amplification of their biological activities.3
Cytokine activity in vivo therefore, must be tightly controlled in order to maintain the homeostasis of the immune system. In keeping with this level of complexity, numerous mechanisms are known to operate in vivo to regulate the activity of cytokines. These mechanisms act at different levels, including: (1) cytokine synthesis and secretion; (2) regulation of the responsiveness of target cells; (3) binding of cytokines to their membrane receptors; and (4) signaling events after cytokine/receptor interaction. Table 2 summarizes information on the different levels and mechanisms that regulate cytokine activity.
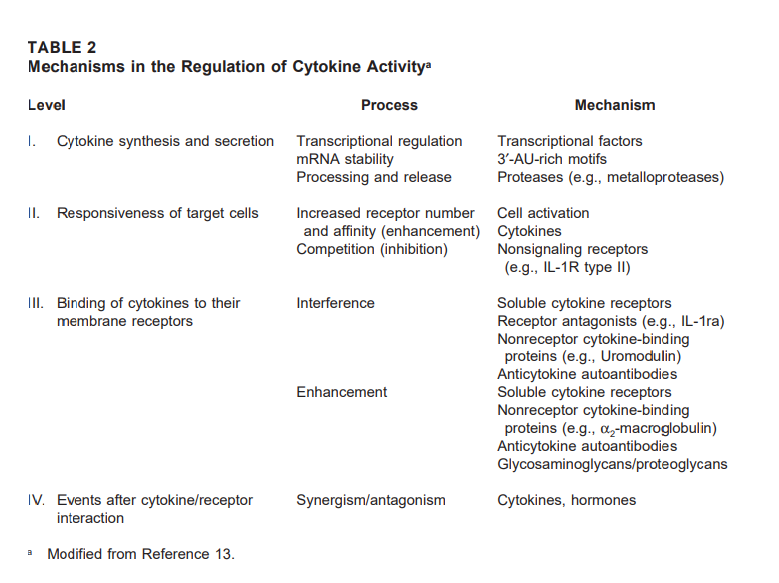
A. Cytokine Synthesis and Secretion
Cytokine synthesis is under the control of a variety of transcriptional and posttranscriptional mechanisms. Most cytokines are not produced constitutively, but are synthesized and secreted in response to cellular stimulation. The types of stimulation that can induce secretion of cytokines depend on the nature of the cytokine-producing cell. For example, mononuclear phagocytes are stimulated by endotoxin and other microbial products, immune complexes, complement components, and contact with activated T cells to synthesize the “proinflammatory cytokines”, TNFα, IL-1, and IL-6.
The major signals that induce the secretion of cytokines from T cells (e.g., IL-2, IL-4, IFNγ) are antigens presented in the context of MHC molecules and mitogens.19,30 Cytokine-inducing signals normally result in the synthesis or activation of transcriptional factors, which directly, but transiently, activate transcription of cytokine genes.31 Moreover, cytokine mRNAs are usually short-lived due to the presence of AU-rich motifs in their 3′ untranslated regions that target them for degradation.32 Thus, cytokine secretion is brief and self-limited. Posttranslational mechanisms (i.e., the proteolytic cleavage of precursor molecules) also regulate the release of certain cytokines, such as TNFα and IL-1.33,3
B. Regulation of the Responsiveness of Target Cells
The responsiveness of target cells to a particular cytokine is dependent on the expression of specific membrane receptors for that cytokine and is directly related to the number and the affinity of those receptors. In many cases, cytokine receptors are constitutively expressed, albeit in low numbers, thus assuring the responsiveness of resting cells to the regulatory effects of cytokines.
Cellular activation normally results in an up-regulation of the number of membrane cytokine receptors and/or an increase in their affinity, thus enhancing the responsiveness of the activated target cells.35–40 For example, cellular activation leads to the transcriptional activation and expression of the IL-2Rα subunit, which in combination with the constitutively expressed IL-2Rβ- and γc chainsresults in the formation of a high-affinity IL-2 receptor, allowing the activated cells to respond to very low concentrations of IL-2.41–43 In contrast, expression of certain types of receptors by a cell may have a negative influence on its responsiveness to that particular cytokine. For example, receptors that are unable to generate any signals after ligand binding, such as the IL-1R type II, may function as “decoys”, preventing binding of the cytokine to biologically active receptors (i.e., the IL-1R type I).44,45
C. Binding of Cytokines to Their Membrane Receptors
There are many factors that can modulate the ability of cytokine molecules to interact with their membrane receptors, either inhibiting or potentiating their biological activity. Among these factors are (1) soluble cytokine receptors; (2) receptor antagonists; and (3) nonreceptor cytokinebinding molecules.
1. Soluble Cytokine Receptors (sCR)
There are many examples documenting the fact that most sCR interfere with the binding of cytokines to their membrane receptors.13 Basically, sCR function by competing with the cell surface receptors for the binding of free cytokine molecules. Hence, they prevent their ligands from reaching the specific membrane receptors and generating a signal, leading to inhibition of cytokine activity (see Figure 1).
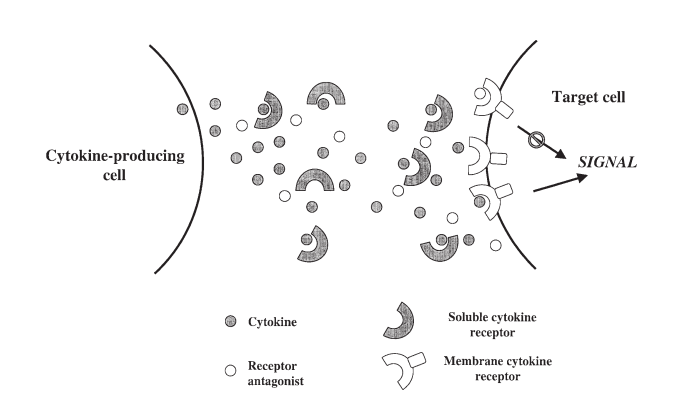
FIGURE 1. Inhibition of cytokine activity by soluble cytokine receptors and receptor antagonists. Soluble receptors bind free cytokine molecules and prevent them from binding to the membrane receptors, whereas receptor antagonists occupy the membrane receptors without generating a signal, thus blocking the active cytokine.
One exception to this rule, however, is that certain sCR can interact in the presence of their ligand, with their signal transducing subunit, thus generating a signal (i.e. the sIL6R and gp130). This type of soluble receptors enhance rather than inhibit the activity of their ligands.46,47
Despite the ability of most sCR to antagonize the binding of their specific cytokines in vitro, a number of them have been reported to potentiate the activity of their ligands in vivo, particularly in situations where the concentration of sCR is not in great excess over that of the cytokine.48 The ability of sCR to enhance the concentrations of active cytokines in vivo is probably the result of several factors, such as increased cytokine stability, decreased proteolytic degradation, and altered pharmacokinetics in vivo (e.g., enhanced half-life, reduced clearance).49–51
Such a function as cytokine “carriers” may help potentiate the systemic or endocrine effects of cytokines. Many different types of sCR have been described both in mice and humans, suggesting that their generation is a common physiologic occurrence. The generation and function of these molecules are discussed in the next section.
2. Receptor Antagonists
A different type of molecules that regulate cytokine activity by preventing their interaction with membrane receptors are receptor-binding antagonists. These molecules are related structurally to the biologically active cytokine and bind to the same receptor, with the difference that they are unable to generate a signal after binding, thus blocking the active cytokine molecule from occupying available receptors (see Figure 1).
One example of this type of inhibitor is the IL-1 receptor antagonist (IL-1ra).52,53 This molecule is produced by monocytes (the same cells that secrete IL-1) and functions as a negative regulator of IL-1 activity in vivo, even though considerably greater concentrations of IL-1ra (when compared with those of IL-1) are required to exert its inhibitory effects.12
More recently, evidence has been presented for the existence of variant forms of several cytokines, including IL-2, IL-4, IL-5, and G-CSF.54,55 These variants lack part of their sequence due to an exon-deletion mechanism mediated by alternative RNA splicing. Some of these molecules may potentially function in the same fashion as receptor antagonists, blocking the binding and activity of the native cytokine, while unable to signal themselves.
3. Nonreceptor Cytokine-Binding Molecules
Besides sCR, several nonreceptor-related proteins are known to have cytokine-binding properties, albeit in a less specific manner, and potentially could contribute to the regulation of their activity in vivo. As in the case of sCR, both antagonistic and “carrier” functions have been reported for such proteins. In some cases, these proteins are able to prevent the binding of cytokines to their membrane receptors, limiting their biologic activity. For example, Uromodulin, an 85-kDa protein present in the urine of pregnant women, can bind both IL-1 and TNFα, inhibiting their activity.56,57
In contrast, α2-macroglobulin, a major protein in serum, has been implicated to act as a transport protein rather than as an inhibitor for several cytokines, including IL-1, IL-2, and IL-6. Such a function may be related to its abilities to both bind cytokines and to act as a protease inhibitor, therefore protecting such cytokines from proteolytic inactivation while transporting them in circulation.58–60 The extent of the contribution of these nonreceptor proteins to the overall regulation of cytokine activity in vivo is still far from clear. Another type of nonreceptor proteins with cytokine-binding ability and potential regulatory function is constituted by natural autoantibodies against cytokines. For example, autoantibodies with specificity for IL-1α and IL-6 have been reported in the serum of normal human donors.61–64 Interestingly, the levels of these antibodies were found to be increased in patients with a variety of rheumatic and other autoimmune diseases, suggesting a putative pathophysiologic role. The relationship of these autoantibodies to human disease has not been clearly established, and their significance remains mostly a matter of speculation.
A different type of cytokine-binding molecules are glycosaminoglycans (GAGs). GAGs are a group of linear heteropolysaccharides consisting of repeating disaccharide sequences in which one residue is an amino sugar, usually D-glucosamine or galactosamine, and the other an uronic acid. Both residues can be sulfated at various positions, contributing to their heavy negative charges.65–67 GAGs exist naturally as nonassociated forms or as covalent complexes with core proteins, or proteoglycans (PGs).65–69 GAGs and PGs are found in almost all tissue types as components of the extracellular matrix, basement and cellular membranes, and mast cell granules.65,67–69 Besides their prominent role of as structural components, GAG/PGs act as co-receptors for a number of growth factors (i.e., fibroblast growth factor [FGF], platelet-derived growth factor [PDGF]); cytokines (i.e. IL-3, GM-CSF, IFNγ); and many chemokines.67,68,70–74 The role of GAGs and PGs in the regulation of cytokine activity has been proposed to involve the immobilization or “tethering” of cytokines to cell surface or basement membranes, avoiding their dilution in extracellular fluids. The bound cytokine molecules can then be presented to specific cytokine receptors on the same or neighboring cells.68,74
D. Signaling Events After Cytokine/Receptor Interaction
Some cytokines can have either synergistic or antagonistic effects toward the activities of other cytokines, without actually affecting expression of their membrane receptors or their ability to interact with them. Such effects might be mediated by potentiation or interference with the signals generated after cytokine/receptor interaction. One example of cytokine antagonism is that between the activities of IFNγ and IL-4 on B lymphocytes, in which IFNγ and IL-4 reciprocally inhibit each other’s activities.1,75 The synergistic and antagonistic relationships among different cytokines appear to be central to the regulation of cytokine networks. However, the biochemical bases responsible for the synergism or antagonism among cytokines at the intracellular level remain to be investigated.
III. CYTOKINE RECEPTORS: MEMBRANE AND SOLUBLE FORMS
The genes encoding most cytokine receptors have now been cloned and expressed, and many of their structural features and signal transduction mechanisms have been elucidated.6–9 Cytokine receptors can be grouped into four different families, based on the presence of conserved sequence homologies, folding motifs, and functional characteristics. These families are (1) the immunoglobulin superfamily, which includes receptors such as those for IL-1; (2) the hemopoietin receptor family, which includes the receptors for most interleukins, CSFs, and several hormones; (3) the TNF receptor family, which includes the receptors for TNFα and other membrane molecules of immunological significance; and (4) the Chemokine receptor family, which includes the receptors for the chemokines and is related to other seven transmembrane helix receptors coupled to heterotrimeric GTP-binding proteins (e.g., β-adrenergic receptors).5 Many cytokine receptors, particularly those that belong to the hemopoietin receptor family, are complexes of two or more different subunits, in which ligand-binding chains (normally the α subunits) associate with specialized signal transducing molecules (normally the β/γ subunits). In many cases, the signal-transducing subunits function as “affinity converters”, increasing the binding affinity of the ligand-binding subunit for its ligand, probably as a consequence of stabilization of the cytokine/receptor complex and/or a reduction in its dissociation rate.37,38 Such “high-affinity” cytokine receptor complexes can have 50- to 1000-fold greater affinities than the ligand-binding subunits alone. As discussed before, evidence from numerous studies now indicates that in addition to their membrane-bound forms, many cytokine receptors, particularly those that belong to the hemopoietin receptor and TNF families, also exist naturally in soluble form. The first description of a soluble cytokine receptor was made by Rubin et al.,76 who discovered the presence of soluble IL-2R molecules (the low-affinity IL-2Rα subunit, or CD25), in supernatants from activated peripheral blood T cells. However, it was not until the systematic cloning of cytokine receptor genes in the late 1980s and early 1990s, and the discovery of alternatively spliced messages encoding soluble forms, that the widespread existence of soluble cytokine receptors became apparent. From a structural standpoint, sCR are truncated versions of their membrane counterparts (in most cases, the ligand-binding α-subunits), lacking the transmembrane and intracytoplasmic domains. Because most of the extracellular domain is conserved in sCR, they retain their ligand (cytokine)-binding ability. Although the affinities of sCR are often comparable to those of their corresponding membrane receptors, there are some instances in which the affinities of sCR are significantly lower than those of the “high-affinity” membrane receptor complexes, where the stability of the binding interaction is enhanced by the transducing subunits. In such cases (e.g., the sIL-2R), considerably high concentrations of soluble receptors are needed to effectively compete with membrane receptors and inhibit cytokine activity. Table 3 summarizes the information on the composition of membrane and soluble receptors for the major cytokines discussed in this review.