Introduction
The defence system of vertebrates uses sophisticated microbial recognition patterns as a defence mechanism against microbial invasion. On the other hand, pathogens exerts their own invasion and pathogenesis mechanism that are (in most cases) capable of overcoming host defence mechanism, and subsequently establishing infection. The immune defence mechanism including the recognition systems for signalling presence of invading pathogens manifests in the form of innate and acquired immunity.
In mammals, these systems have been molecularly characterized, with details of the acquired immune system having first emerged from studies performed on ancient cartilaginous fish (Flajnik & Kasahara 2001). The acquired immune system is affected through actions of highly variable peptide-antigen receptors, such as immunoglobulins and T-cell receptors.
Innate immune response, on the other hand, involves dendritic cells (DCs) and pattern-recognition receptors (PRRs), with activation precedes that of acquired immune system. Attention to PRRs, have now been captured due to recent elucidation of the ligand properties of toll-like receptors (TLRs) and TLR-mediated DC maturation.
Dissection of the mechanism of TLR-mediated DC maturation has promoted our current understanding of TLRs and its mechanism of action. TLR-mediated DC maturation facilitates driving of effector cells by maturation of antigen (Ag)-presenting DCs. This is in turn, determining the nature of antigen towards which immune cells are proliferated whereas microbial patterns determine which effectors are selected for immunological output (Seya & Matsumoto 2009).
By means of a complex receptor array, Natural killer (NK) cells can recognize variable patterns of ligands and regulate or amplify their effector functions (Fig. 1).
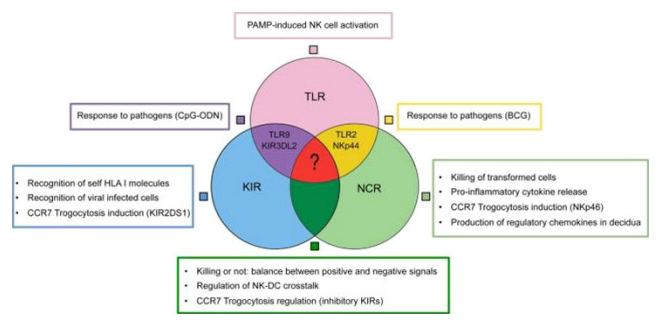
Figure 1 A wide and heterogenous group of receptors allow NK cell to fulfil multifaceted functions. The nodes of this intricate functional network may represent new therapeutic targets in different pathological condition.
Such NK receptors include old, rather conserved, molecules, such as TLRs, which enable NK cells to respond both to viral and bacterial products. That also extended to include newer and evolving molecules, such as killer Ig-like receptors and natural cytotoxicity receptors, which control NK cytotoxicity, and therefore, are responsible for the elimination of virus-infected or tumour cells.
In addition, rapidly gained new functions, NK cells can also acquire new receptors by trogocytosis. Thus, NK cells may have adapted their receptors to different functional needs making them able to play a key role in the modulation of critical events occurring in several cellular compartments (Sivori, Carlomagno, Pesce, Moretta, Vitale & Marcenaro 2014). Toll-like receptors recognize pathogen-associated molecular patterns (PAMPs), which are characteristics of microbial structures, inducing antimicrobial responses (Fig. 1).
In human genome, 10 TLR members have been identified with their functions were determined from analyses of TLR-deficient mice (Takeda, Kaisho & Akira 2003): the TLR2 subfamily recognizes bacterial cell wall peptidoglycan (PGN) and acylated lipopeptides, TLR4 recognizes Gram-negative bacterial lipopolysaccharides (LPS), TLR5 recognizes bacterial flagellin, while TLR3, 7, 8 and 9 recognize microbial nucleic acids. Mouse TLR stimulation results in signal transduction through one of the five adaptor proteins, MyD88, TIRAP/MAL, TICAM-1, TICAM-2 and SARM (Liew, Xu, Brint & O’Neill 2005). With the exception of TLR3, the adaptor molecule for signalling in all TLRs studied to date is the MyD88. MyD88 recruits members of the interleukin-1 receptor-associated kinase (IRAK) family which in turn activate the key ubiquitin E3 ligase, tumour necrosis factor receptor-associated factors (TRAFs) and transforming growth factor-a (TGFa)-activating kinase (TAK1). This in turn leads to the activation of the transcription factor nuclear factor-jB (NF-jB) (Liew et al. 2005). The activated NF-jB is translocated to the nucleus producing an expression of inflammatory cytokine genes such as IL-1b and IL-6. In contrary, TLR3 recruits the adaptor molecule TICAM-1, leading to activation of the interferon (IFN) regulatory factor-3 (IRF-3) and induces type I IFN and IFN-inducible genes. The MyD88 pathway is conserved in a wide range of vertebrates, but whether or not the TICAM-1 pathway is conserved in lower vertebrates is still controversial (Seya, Matsumoto, Ebihara & Oshiumi 2009). Comparing the TLR families across the vertebrate species has revealed that water-living vertebrates possess TLR14, 21 and 22, in addition to the mammalian-type TLRs (Oshiumi, Matsuo, Matsumoto & Seya 2008). The TLR repertoire was established during evolution in a species-specific manner in jawed vertebrates, which also depends on the respective living environment, whether it is aquatic or land (Oshiumi, Tsujita, Shida, Matsumoto, Ikeo & Seya 2003; Ishii, Kawasaki, Matsumoto, Tochinai & Seya 2007; Higuchi, Matsuo, Shingai, Shida, Ishii & Funami 2008). On the other hand, some invertebrates such as the sea urchin and amphioxus possess a large number of TLRs that were identified through their respective genome projects (Huang, Yuan, Guo, Yu, Li & Wu 2008). Some exceptions exist such as in the case of Ciona intestinalis which has only 2 functional TLRs (Irvine, Carr, Bailey, Kawasaki, Shimizu & Amemiya 2002). When compared side-by-side, the fish TLRs and the factors involved in their signalling cascades have high structural similarity to their mammal counterparts. Nonetheless, the fish TLRs exhibit very distinct features with large diversities that reflect the evolutionary history, and the distinct environments that they occupy. Of at least 16 TLR types identified in fish, direct evidence of ligand specificity has only been shown for TLR2, TLR3, TLR5M, TLR5S and TLR22 (Fig. 2). Any future researches to assumingly focus on specific aspects regarding fish TLRs. That include the identification of specific ligands of all fish TLRs that will ultimately led to better understanding of the disease resistance mechanisms in fish and the development of new adjuvants and/or more effective vaccines and therapeutics (Palti 2011). At present, a large part of our knowledge on the fish immune system comes from studying fish species important for aquaculture. Traditionally, the most studied species include common and crucian carp (both Cypriniformes), channel catfish (Siluriformes), rainbow trout and Atlantic salmon (both Salmoniformes), tilapia, sea bass and seabream (all Perciformes), Japanese flounder (Pleuronectiformes) and Atlantic cod (Gadiformes). Increasingly, the knowledge on the fish immune system is substantiated by information from fish species of economic importance to Asia, including grass carp and Indian carp (Cypriniformes) and pufferfish (Tetraodontiformes), but also the miiuy and the yellow croaker as well as the orange-spotted grouper (all Perciformes). Further, not to be underestimated, there are a number of freshwater fish species that have become important experimental models in immunology, including medaka (Oryzias latipes, Beloniformes/Cyprinodontiformes), stickleback (Gasterosteus aculeatus, Gasterosteiformes) and zebrafish (Cypriniformes). Although the list of fish species studied for their immune system still is increasing, it may also be clear that our knowledge of fish immunology is built on only a minor fraction of the approximate 27,000 fish species known. We therefore should not be surprised if the organization of the immune system is not exactly the same across fish species. However, in general, the closer the phylogenetic relationship between fish species, the closer the similarities (Pietretti & Wiegertjes 2014).