1. introduction
Fish are in intimate contact with their environment, which can contain very high concentrations of bacteria and viruses. Many of these are saprophytic, some are pathogenic and both are very capable of digesting and degrading the fish's tissues. However, under normal conditions the fish maintains a healthy state by defending itself against these potential inva- ders by a complex system of innate defense mechanisms. These mechanisms are both constitutive and responsive (i.e. pre-existing or inducible) and provide protection by preventing the attachment, invasion or multiplication of microbes on or in the tissues.
Their importance is three-fold. Firstly, the protection is non- specific and does not depend upon recognition of the distinctive molecular structure of the invading species. Secondly, there is no or only little time lag for them to act. Even the inducible defenses, like inflammation, are relatively quick to respond (1-2 days) and thus give pathogens little time to establish themselves. Thirdly, they are relatively temperature independent. For ectothermic vertebrates, these characteristics are very important because the specific immune defenses take a considerable time to respond and are very temperature-dependent.
For temperate species like salmonids, antibody production takes at least 4-6 weeks even at optimum tempera- tures and when many pathogens can kill the fish within a few days of infection, the protection afforded by the specific response is important only in previously immunised fish. Thus it is to be expected that the innate defenses might be more important in fish than in endothermic vertebrates and indeed some of them like lysozyme and complement appear to be more potent in fish than in mammals.
2. Viral diseases
Viral diseases cause very significant losses in aqua- culture. Some viruses mainly affect young fish e.g. Infectious Pancreatic Necrosis Virus (IPNV) in salmonids, while others cause mortality in fish throughout their life e.g. Viral Hemorrhagic Septice- mia virus (VHSV) and Infectious Salmon Anemia virus (ISAV).
Many fish viral pathogens produce a persistent carrier state in the host. These fish can shed virus into the environment and be a source of infection to other fish. Low levels of virus can often be isolated from the kidney of carrier fish and evidence indicates that some viruses e.g. IPNV, can replicate in and be released from the kidney macrophages without being cytolytic [1].
Little is known about the mechan- isms involved in viral carrier states in fish and how the viruses evade the host defenses. The innate defenses against viruses in fish may be categorized as constitutive or responsive.
A constitutive innate anti-viral response in fish comprises the action of non-specific cytotoxic cells to virus-infected cells, and a responsive mechanism is interferon production, which is non-specifically inducible by virus infection. In some cases, e.g. defense against the rhabdoviruses, VHSV and IHNV, complement appears to play an essential role in virus neutralization mediated by the specific antibody response.
Other constitutive defenses may include factors like lectins (which could bind to glycosylated residues on the surface of viruses), and non-specific lysins (which could lyse the envelopes of enveloped viruses) but evidence for these is lacking in fish. For viruses to replicate in fish cells they first have to attach to the surface of the cell, enter the cell membrane and engage the cell's biochemistry for nucleic acid and protein production. All of these stages will require molecular recognition and if these are lacking the cell will be refractory to viral replication. Very little is known about virus/host recognition for viral diseases of fish though some progress is being made with VHS and ISA.
2.1. Interferon and Mx proteins.
The type I interferon system is a rapid and powerful antiviral defense mechanism in vertebrates [2]. Inter- ferons (IFNs) are pH-resistant cytokines which are produced by many cell types in response to a viral infection. Viral double-stranded RNA (dsRNA) induces the production of IFN and the synthetic dsRNA polyinosinic polycytidylic acid (poly I:C) is a very potent inducer of IFN. Most viruses produce dsRNA at some time in their replication [3] and it appears that animals have evolved an ability to recognise these molecules and to respond to them by this innate mechanism.
In mammals, the mode of action of IFN is to induce the expression in host cells of a number of proteins which inhibit the translation of viral mRNA. These proteins include 2',5'-oligoadenylate synthetase, protein kinase P1 and Mx proteins [4]. IFN-like activity has been demonstrated in a number of fish species [5] but to date neither the protein nor the genes have been isolated. However, the genes encoding the Mx protein have been cloned for several fish species, including rainbow trout [6-8], Atlantic salmon [9] and Atlantic halibut [10].
Detection of the expression of Mx gene mRNA by RT-PCR [9] or of Mx protein using labelled antibodies [7,8,11] have been used as a sensitive method for detection of INF responses in fish. Production of IFN-like activity and Mx gene/ protein expression in fish has been demonstrated in vitro and in vivo in response to a number of viruses as well as poly I:C (see Table 1).
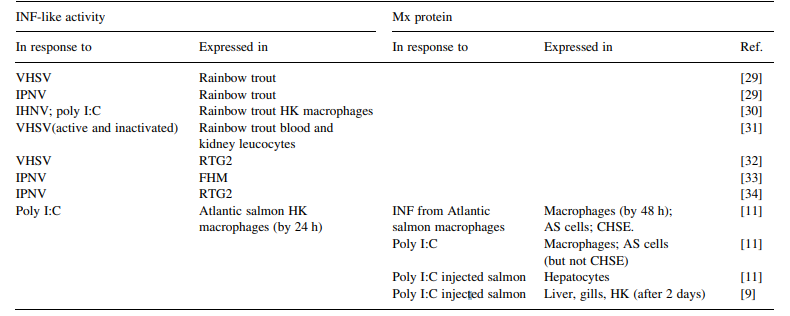
Table 1 Production of Interferon-like activity and Mx protein by fish and fish cells. Abbreviations: AS Atlantic salmon cell line: CHSE chinook salmon embryo cell line(this cell line is unable to produce INF in response to virus or stimulation with Poly I:C but is able to respond to INF and produce Mx protein and be protected from viral infection, [11]; HK head kidney; FHM fathead minnow cell line; IHNV infectious hematopoietic necrosis virus; IPNV infectious pancreatic necrosis virus; VHSV viral hemorrhagic septicemia virus
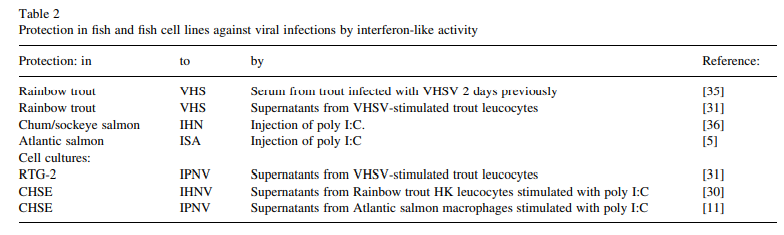
Protection against infection of fish and fish cell lines against several viruses has been demonstrated by injection of, or exposure to IFN-containing serum or supernatants, respectively, (see Table 2), Rainbow trout appear to have three Mx genes and proteins [6-8] while Atlantic salmon and halibut appear to have two [9.10]. The molecular weight of the trout Mx proteins is about 70 kDa [8] and of salmon about 76 kDa [11].
Interferon production occurs very rapidly after virus infection (within 2 days in rainbow trout injected with VHSV: [12] and occurs in very young fish (rain- bow trout fry of less than 0.2 gm, 600 degree days; [13]). In isolated Atlantic salmon macrophages stimu- lated with poly LC, peak interferon production occurred within 24 h and peak Mx protein production after 48 h [11]. In Atlantic salmon injected with poly I:C, Mx protein was produced by various tissues within 2 days and for at least 14 days [9].
Thus, IFN-mediated antiviral defense mechanisms are able to respond during the early stages of a viral infection and this has led many authors to believe that IFN responses provide some degree of protection until the specific immune defenses are able to respond. However, serum IFN levels in rainbow trout follow- ing experimental infection with VHSV do not corre- late with resistance and more closely reflect the level of viraemia ie it is the most susceptible fish which show the highest IFN responses (see below). Recent work with DNA vaccines against VHS and IHN has shown that they not only stimulate the production of antibodies and protection but they also induce the expression of Mx genes in the muscle tissues of the rainbow trout at the site of injection [13]. Interestingly, the control DNA plasmid lacking the viral glycoprotein gene insert did not induce any of these responses. Work in the author's laboratory has confirmed this. Furthermore, protection against VHS can be found as early as 1 week after DNA vaccination, a time when Mx genes were already being expressed, while antibodies to the VHSV were not detected until between 4 and 8 weeks post- vaccination (McLauchlan, unpublished). It thus seems likely that DNA vaccines may induce a rapid protection against viruses, which is mediated by the innate non-specific IFN responses while long-term protection is mediated by the specific immune responses.
2.2. Anti-viral cytotoxic cells
Although it is well established in higher vertebrates that natural killer (NK) cells are important in limiting the spread of virus by lysing infected cells in the early stages of infection, very little work has been done to investigate this aspect in fish. However, it is apparent that such mechanisms do exist in fish. Kidney leuco- cytes of normal rainbow trout and Atlantic salmon, while showing some in vitro cytotoxicity for RTG-2 and AS cells (tissue culture cell lines derived from rainbow trout and Atlantic salmon, respectively), were significantly more cytotoxic when the cell lines were infected with IPNV [14,15).
Because NK-like activity is shown by leukocytes in assays using allo- geneic cells, even when the target cells are not infected with virus, clearer evidence for anti-viral cytotoxic cells was provided using a channel catfish autologous system [16]. Here, autologous catfish lymphoid cell lines, which were experimentally infected with Channel Catfish virus, were killed by peripheral blood leukocytes from the same (non- immunized) individual fish, while non-infected auto- logous target cells were not.
Furthermore, the results of these workers indicated that the cytotoxic cells responsible for killing allogeneic targets were a differ- ent population from those responsible for killing viral- infected targets. These workers also showed that blocking late viral gene expression in the infected target cells with acycloguanosine did not affect the level of cytotoxicity indicating that expression of early viral gene products was sufficient to render the target cells susceptible to lysis. Thus, the defensive power of these cytotoxic cells is clear, as they are capable of destroying infected cells even before the entire viral genome has been expressed to produce new infective particles.
2.3. Miscellaneous innate anti-viral defenses
2.3.1. Glucan-induced
trout injected ip with glucan were more resistant to immersion challenge with IHNV than saline injected controls [17]. This increase in resis- tance took about 3 weeks to develop and lasted for up to 58 days. The mechanism of this resistance is not understood but it did not correlate with serum neutra- lising antibody production which was, paradoxically, lower in the serum of surviving glucan-injected fish than the controls.
2.3.2. Complement
The ability of salmonid antibodies to neutralize the rhabdoviruses, VHSV and IHNV, is dependent on the presence of complement [18]. Results indicate that the classical complement pathway is involved but attempts to demonstrate the involvement of C3 have not been successful and the full mechanism of the role of complement is still unknown [19]. It is possible that the requirement for complement is related to the enveloped nature of rhabdoviruses and depends on the membranolytic activity of comple- ment lysing the virus particle. Non-enveloped viruses e.g. IPNV do not require complement for antibody to neutralize them.
2.3.3. Genetic resistance
There are strong indications that genetic traits are involved in resistance of rainbow trout against VHS. Using gynogenetic reproduction it has been possible to obtain highly resistant families of trout after only two generations [20]. Brook trout (Salvelinus fontina- lis) are highly resistant to VHS and this resistance is transmitted to triploid hybrids between rainbow trout dams and brook trout sires [12]. When hybrids and rainbow trout were challenged with VHSV by either immersion or injection, mortalities were much lower in the hybrids. Surprisingly, while viraemia was lower in the hybrids, so was the interferon responses. Inter- feron levels reflected the level of viraemia, both of which were high in the susceptible rainbow trout, as well as the few moribund and dead hybrids. Thus, there was no correlation between resistance and inter- feron responses, in fact the opposite was apparent. Other attempts to identify the mechanism of resist- ance against VHS have focussed on comparisons of allelic variations in the complement component C3 as well as MHC between resistant and susceptible families of rainbow trout [21,22] but once again with no success. The basis of this genetically determined VHS-resistance in rainbow trout has thus still to be elucidated.
2.3.4. Host/virus compatibility
The integumental tissues of fish are important as the portal of entry of viruses and they may be considered as a barrier to infection in resistant fish or as a primary site of attachment and replication in susceptible fish. This hypothesis was tested [23] in two strains of rain- bow trout, one which was susceptible to VHS by both immersion and injection challenge and another which was only susceptible to injection challenge. A strain of rainbow/brook trout hybrid, which was resistant to both routes of challenge, was also studied. Pectoral fins, gills and skin were excised and immersed in a VHSV suspension for 1 h.
To assess differences in viral adsorption, the tissues and supernatant were titred for virus. Titres in the tissues were very low, indicating that only very few particles had attached to the tissues, and no differences were detected between the tissues from different fish. This was confirmed in the supernatants, which showed no decrease in the original titre. Replication of virus in the excised tissues was assayed after 3 days of culture. Viral multiplication in all the tissues excised from suscep- tible trout was very high while there was no indication that virus replication occurred at all in any of the hybrid tissues. In trout that were susceptible to injec- tion challenge only, replication of VHSV occurred only in the skin. A possible interpretation of this is that the pieces of skin used were composed not only of epidermal layers that could be resistant to viral repli- cation, but also inner layers of dermis and blood vessels in which virus could have been able to repli- cate. Thus, the susceptibility of different host cells to support viral replication may reflect different levels of resistance in fish to VHS. Recently, progress has been made in understanding the mechanisms of how ISAV attaches and enters Atlantic salmon cell lines. This virus attaches to sialic acid residues on the surface of the host cell, leading to endocytosis of the virus by the cell. Fusion of the virus with the host cell membrane takes place in the endo- some and is dependent on low pH. The viral genome is then released into the cytosol of the cell [24]. It is known that large structural diversity occurs in sialo- glycoproteins [25] and certain types of sialoconju- gates appear to be unique to salmonids [26]. This is relevant because it is known that such host cell surface receptors are major determinants of host range and tissue tropism for many viruses of higher vertebrates [27]. Thus, the precise nature of the type of sialic acid residues recognised by ISAV may provide a means of identifying more or less resistant salmon for selective breeding purposes.
2.4. Conclusions
It is particularly evident from studies on VHS in rain- bow trout that innate defenses can play a very important role in resistance to viral infections but we have virtually no understanding of the mechanisms involved. While few studies have yet been performed to correlate resis- tance with innate defense responses, serum interferon responses and differences in C3 and MHC genes do not appear to explain differences in resistance to VHS. No studies have yet been performed to find correlates with anti-viral cytotoxic cells. It has long been recognised that many viral diseases have a limited host range even amongst very closely related viruses. For example, marine strains of VHSV are not virulent or have low virulence in rainbow trout though virulence can be increased by repeatedly passaging the virus through rainbow trout [28]. It seems possible that innate differences in susceptibility of fish to different viruses resides in differences in the ability of viruses to attach to, enter or replicate in different fish host cells. It would, therefore, be of interest to study the ability of different fish cells isolated in vitro (especially superficial tissues that are likely to be the first site of viral adsorption and multiplication), to support viral replication and produce interferon and Mx protein. Finally, much more work is required to identify the molecular recер- tors on the surface of susceptible fish cells which may allow means of modulating their structure or avail- ability to the virus or identifying resistant strains of fish for selective breeding.
3. Bacterial diseases
The innate defense mechanisms of fish against bacteria include production of broad-spectrum antimicrobial substances and acute phase proteins, non- classical complement activation, release of cytokines. inflammation and phagocytosis. The nature and mechanisms of many of these defenses are reviewed in other articles of this volume. While these mechanisms provide potent defenses against invasion against saprophytic environmental bacteria, pathogenic bacteria have evolved means of avoiding many of them.
In some cases, non-immunised fish appear to co-exist with highly virulent bacterial pathogens in a carrier state, without showing any signs of morbidity [37], so presumably the innate mechanisms of defense provide some degree of protection. However, disease out-breaks and mortalities often result from the fish being stressed and presumably these defense mechan- isms being compromised. Very little is known or understood about such interactions.
3.1. Integumental innate defenses
Many observations suggest the importance of innate defenses in the integument of fish. For instance it is well known that rainbow trout are virtually resis- tant to immersion challenge by Aeromonas salmoni- cida while Atlantic salmon are very susceptible. However, there is little difference in the LD50 when these fish species are injected with the bacteria [38].
3.1.1. Mucus
The protective role of skin mucus has been demon- strated by carefully removing mucus with a swab and then challenging turbot [39] or ayu [40] with Vibrio anguillarum and inducing increased mortality. The precise mechanisms of this protection are not known and are probably multifactorial. Mucus is continually being produced and sloughed from the integumental surface thus physically trapping and preventing bacteria from attaching to the epithelium and having an opportunity to invade the fish's tissues. Mucus also contains many substances with antibacterial activities and these may play important roles.
3.1.2. Anti-bacterial peptides
These substances have been identified from mucus secretions of a number of fish species [41] but little is yet known about their ability to kill fish-pathogenic bacteria. Pathogenic strains of A. salmonicida are less susceptible to cecropin P1 (an antibacterial peptide derived from the pig) than non-pathogenic strains lacking the A-layer, but they were, nevertheless, killed by higher concentrations [42]. The gene encod- ing the anti-bacterial peptide pleurocidin has been cloned from the winter flounder and shown to be predominantly expressed in skin and intestinal tissue and the gene is first expressed as early as 13 days post- hatch [86]. Thus these peptides may provide an important line of defence before development of the specific immune response in larval fish. Synthetic pleurocidin has recently been shown to protect coho salmon from infection by V. anguillarum [87].
3.1.3. Proteases
Trypsin-like proteases and cathepsin L and B proteases have been found in skin mucus of a number of fish species [43]. The ability of these enzymes to lyse formalin-killed V. anguillarum has led to the suggestion that they may play a role in defense against bacteria but their action on live bacteria does not appear to have been studied yet.
3.1.4. Lectins
Once bacteria have made contact with their fish host, many pathogenic species can adhere to the mucus and the epithelial cells by surface molecules known as adhesins [44]. Frequently, these interactions involve binding of carbohydrates. Once the bacterium has attached to the host cell, the latter is induced to endocytose the bacterium which can then grow and spread in the host to produce disease [45]. Lectins are a group of proteins with different speci- ficities for binding carbohydrates [46]. They have been found in salmon eggs [47], serum [48-50] and mucus [51]. These lectins are Ca²+-dependent and can agglutinate a number of fish bacterial pathogens. Their role in defence is still unclear but in mammals they can have opsonic and complement-activating proper- ties [46]. It is possible that an important role of lectins in fish mucus is to bind to the carbohydrates on the surface of bacteria which are involved in attachment to the integumental cells. This could block attachment and subsequent invasion of the host, but such experi- ments have not yet been done.
3.1.5. Lysozyme
This enzyme can attack the peptidoglycan layer of bacterial cell walls causing them to lyse. Lysozyme has been found in fish mucus, serum and ova [52]. Peritoneal macrophages and blood neutrophils contain lysozyme and the latter are thought to be the source of serum lysozyme [84]. The production of lysozyme by Atlantic salmon macrophages in vitro is enhanced in the presence of yeast glucan or bacter- ial lipopolysaccharide [85]. Fish lysozyme occurs in two forms and one of these appears to be much more bactericidal than lysozyme of higher vertebrates [94]. There are several reports of lysozyme isolated from fish serum and ova, being bactericidal even for important fish pathogens like A.salmonicida and A. hydro- phila (see Ref. [52]).
3.2. Systemic innate humoral defenses
If bacteria are successful in crossing the integumental defenses there are a number of plasma proteins which may prevent further spread of the infection. Some of the proteins mentioned above as present in the mucus also occur in higher concentration in the serum, e.g. lysozyme and lectins. However, probably the most important of the serum defense factors is the complement system because of its activating effects on the cellular defenses.
3.2.1. Complement
The complement of teleost fish can be activated directly by lipopolysaccharide (LPS), which is a major constituent of the cell wall of Gram-negative bacteria. This is the so-called alternative complement pathway (ACP) and results in lysis of the cell membrane of many non-virulent bacteria. However, the species of bacteria that cause disease in fish are resistant to being killed by this mechanism [52], though some can be killed when the complement is activated by the classical (antibody-mediated) path- way, e.g. V. anguillarum serogroup Ol 153,54). Nevertheless, complement has another important innate defense function. During its activation on the bacterial cell wall, two components are important for recruiting phagocytes. The CSa component is released from the complement complex and is a potent chemo- taxin for macrophages and neutrophils. These cells have receptors for the C3b component which remains attached to the bacteria, which are then more readily phagocytosed (see below). The ACP activity is very high in fish serum compared with mammals [55] suggesting this pathway is very important in the defense mechanisms of fish.
3.2.2. Lectins
As mentioned above, lectins have been isolated from the serum of a number of fish but evidence for their role in defense is only recently coming to light. A mannose-binding lectin, isolated from the serum of Atlantic salmon, has been shown to have opsonising activity for a virulent strain of A. salmonicida and furthermore, lectin-coated bacteria induced the macrophages to produce an enhanced respiratory burst and were more susceptible to being killed by the macrophages [82]. An N-acetyl-galactosamine-binding lectin has been isolated from the serum of blue gourami [50]. This lectin was shown to have opsonising activity and lectin-treated virulent Aeromonas hydrophila cells were killed in the presence of complement [83]. Furthermore, supernatants obtained from lectin- stimulated macrophage cultures exhibited significant bacterial-killing activities [83].
3.2.3. Pentruxins: C-reactive protein (CRP) and serum amyloid protein (SAP)
These serum proteins are usually acute-phase proteins in mammals but in fish they appear to be consitutively expressed and may show only a slight increase or decrease in concentration during inflam- matory responses [56.57]. Pentraxins are capable of binding to a number of polysaccharide structures in the presence of Ca" ions. Their role in defense is not well understood but in mammals they are capable of activating complement and phagocytes have receptors for them. While there are many reports of the presence of pentraxins in the serum, mucus and ova of fish there is only one report of CRP binding to bacteria (V. anguillarum) and activating complement with enhanced phagocytosis [58]. From the widespread occurrence of pentraxins in fish and the relatively high concentrations in serum (50-300 µg/ml; [56]), it is to be expected that they play an important role in defense mechanisms and more research is merited.
3.2.4. Bacterial growth inhibitors
3.2.4.1. Transferrin and the hypoferraemic response.
Bacteria, like other cells, require iron as a co-factor for many enzyme systems but in the host the availability of iron is highly restricted by being bound to the high-affinity iron-binding protein, transferrin, in the plasma. Most bacteria are thereby unable to grow in the host tissues. However, patho- genic bacteria have evolved several ways of overcom- ing this defense by producing high-affinity iron- sequestering mechanisms of their own [52]. Never- theless, as a further step to reduce the availability of iron to pathogenic bacteria, vertebrates show a hypo- ferraemic response. In mammals, the LPS of Gram- negative bacteria results in the release of interleukin-1 (ILI) from macrophages and this stimulates neutro- phils to release lactoferrin. The latter removes iron from plasma transferrin forming lactoferrin-iron complexes that are rapidly sequestered by the liver [59.60]. In fish there is no evidence to date of the existence of lactoferrin but the presence of an iron- binding activity at low pH in lysates of Atlantic salmon leucocytes suggests that it is present [61]. However, the ability of fish to express the hypoferrae- mic response following injection of LPS has been clearly demonstrated [62,63]. Furthermore, bovine lactoferrin has been shown to enhance the respiratory burst of rainbow trout macrophages in vitro [88] and to increase resistance to bacterial and infection after oral administration [89]. A novel role of transferrin in fish has recently been reported. During mixed lymphocyte reactions or mito- genic stimulation of goldfish kidney leukocytes, proteolytic fragments of transferrin were released and these fragments, but not the full length transferrin, were able to induce the production of nitric oxide by LPS-stimulated goldfish macrophage cultures [90]. Thus, as monocytes infiltrate inflammatory sites, transferrin-derived peptides may initiate the differen- tiation of these cells into mature tissue macrophages with enhanced bactericidal properties.
3.2.4.2. Anti-proteuses.
For hacteria to invade and grow in the fish tissues they need to digest host proteins as a source of amino acids. Fish plasma contains a number of protease inhibitors, principally al-anti-protease, a2-anti-plasmin and a2-macroglobulin (a2M) which may play a role in restricting the ability of bacteria to invade and grow in vivo. However, once again it appears that highly adapted pathogenic bacteria have evolved evasive mechanisms, For example, the highly toxic serine protease produced by A. salmonicida is resistant to al-anti-protease. This is regarded as a universal serine protease inhibitor and represents over 80% of the anti-protease activity in salmonid plasma. Nevertheless, the A. salmonicida serine protease is inhibited by a2M [64]. The ability of salmonid plasma to inhibit this bacterial protease has been correlated with between species differences in susceptibility to furunculosis [65.66] and within species differences in rainbow trout [67].
3.3. Systemic innate cellular defenses
3.3.1. Inflammatory response and phagocytes
If bacteria gain entry into the tissues of the fish an inflammatory response is induced with the ultimate influx of phagocytes which have potent bactericidal properties. The inflammatory response in the perito- neal cavity of rainbow trout following the intraperito- neal injection of bacteria has been studied in detail [68-71]. The resting peritoneal cavity contains a population of leucocytes comprised of macrophages (about 40%), lymphocytes (about 55%) and neutro- phils (about 2%). Following injection of bacteria, the macrophage population rapidly phagocytosed the bacterial cells. Over the next 24-48 h, there was a marked increase (500-fold) in the number of neutro- phils in the cavity and a more modest increase in the number of macrophages (eight-fold increase by 96 h). By 48 h, neutrophils were about five times more numerous than macrophages. Neutrophils were also highly phagocytic if any bacteria were remaining at the time they entered the peritoneal cavity from the circulation. Once the bacteria were cleared this response subsided and the resting population level of peritoneal cells was re-established by about 15 days. Of particular interest in these studies was the observation that following the infiltration of neutro- phils into the peritoneal cavity, many of the macro- phages now contained myeloperoxidase and glycogen granules derived from the neutrophils. Resting macro- phages do not contain these substances and the authors speculated that the macrophages could make use of these neutrophil components to enhance their own bactericidal activity. This possible cooperation between macrophages and neutrophils at the site of an in vivo inflammatory response has been omitted from all the experiments dealing with the ability of these cells to kill bacteria in vitro and some of the data describing the resistance of some pathogenic bacteria to macrophage killing e.g. virulent A. salmonicida [72] and V. anguillarum serogroup 02a [73] may have under-estimated the killing power of macro- phages and neutrophils combined.
3.3.2. The control of inflammation
The initiation of inflammation is highly complex and multifactorial. A number of blood enzyme systems, including the clotting system, the kinin system and the complement system play a major role and while little is known of the details in fish it is clear that they share many similarities to their mammalian counterparts [74]. For example, during the activation of the complement system by bacteria (directly by the alternative pathway or indirectly by lectins or CRP) the anaphylactic factors C3a and C5a are produced (75). In mammals, these factors induce the release of vasoactive amines (histamine or 5- hydroxytryptamine: 5-HT) from platelets and mast cells. In fish, thrombocytes and eosinophilic granular cells (EGCs) probably play an equivalent role, though histamine does not appear to be present in fish and the observed degranulation of EGCs by bacterial products may result in the release of 5-HT [76,95,96]. The amines induce local vasodilatation and extravasation of neutrophils and monocytes into the infected site. The C5a component of the activated complement also has chemotactic activity for fish phagocytes [75] and thus they accumulate at the site of infection. This influx of phagocytes is further stimulated by cytokines and eicosanoids. In mammals, bacteria and LPS stimulate macrophages to secrete interleukin-1 (IL1) which sequentially stimulates the release of eicosa- noids, which have pro-inflammatory and chemotactic activity [77]. In fish, a similar process is apparent as LPS has been shown to induce IL-1 production by trout leukocytes [78] and the production of eicosa- noids(with leukocyte chemotactic activity) by a vari- ety of leukocytes has been reported [79].
3.3.3. Phagocytosis
The phagocytosis of bacteria by fish macrophages and neutrophils first requires attachment of the bacteria to the surface of the phagocyte. This may involve hydrophobic interactions or sugar/lectin inter- actions [74]. However, the most active promoter of phagocytosis is the C3 component of complement, which is bound to the bacterial surface LPS directly via the alternative pathway or indirectly via lectins or CRP [74]. The mannose-binding lectin of Atlantic salmon is able to opsonise A. salmonicida even in the absence of complement [82]. 3.3.4. Phagocyte bactericidal mechanisms Fish macrophages and neutrophils both produce bactericidal reactive oxygen species (ROS) during the respiratory burst on contact with or during phago- cytosis of bacteria [74,80]. Neutrophils contain large amounts of myeloperoxidase, which in mammals is involved in the production of bactericidal hypohalite ions (principally from iodine) and presumably the same can occur in fish [52]. Another bactericidal mechanism, is the production of nitric oxide (NO) [91]and subsequent peroxynitrite and hydroxyl ions by fish macrophages [74]. An interesting observation is that following ip. injection of bacteria (Renibacter- ium salmoninarum) into rainbow trout, the kidney showed expression of nitric oxide synthase (the enzyme which produces NO) but a more rapid and sustained response was detected in the gill tissue [81]. This indicates that the gill tissue is capable of mounting a strong bactericidal response, but the cell type (presumably macrophages) has not yet been identified.
3.4. Conclusions
It is plainly evident that innate mechanisms of defense play a vital role in preventing bacterial diseases in fish, yet there is still much to understand. An interesting situation is in the embryos and larvae of fish which do not have a specific immune system. When larvae of Atlantic salmon were challenged with Yersinia ruckeri 2 weeks after hatching, mortalities reached only 8%. As the fish aged they became more susceptible with mortalities reaching 13% when challenged at 4 weeks post-hatch and 60% when challenged from 6 weeks onwards [92]. A simi- lar greater resistance of very young salmonids to furunculosis has also been noted [93]. This strongly suggests that some unidentified factors originating from the mother provided considerable protection against bacterial challenge, and this declined as the larvae aged. Eggs are rich in several non-specific defense factors, like lectins and lysozyme (the source of the latter probably being the ovary) [97], and macrophages differentiate early in embryonic devel- opment (98). It is important to know more about these defense mechanisms and if the health status of the mother might affect the survival rates of her eggs and larvae. Such considerations are important for both farmed and wild fish populations.