Role of skin mucus components in innate immunity
The major function of the mucus appears to provide the effective separation between internal and external environment of fish, abrasion resistance and primary defense against the numerous aquatic pathogens by virtue of its components which include proteases, AMPs, lysozyme, CRP. These are discussed in detail below.
Proteases
Protease refers to a group of enzymes whose catalytic function is to hydrolyze peptide bonds of proteins and mucus of fish contains a variety of proteases which have a significant role in the innate immune mechanisms (Ingram, 1980).
catalytic 촉매의
These proteases are often responsible for degrading pathogens and other foreign substances. The proteases are broadly classified into four categories such as serine proteases, cysteine proteases, aspartic proteases and metalloproteases depending upon the chemical groups responsible for catalysis (Hartley, 1960).
degrade 분해되다
broadly 대략
Serine protease is reported as one of the major mucus proteases in several fish species such as Cirrhinus mrigala, Labeo rohita, Catla catla, Rita rita and Channa punctata and comprises more than 25% of the complement system (Nonaka and Miyazawa, 2002; Nigam et al., 2012).
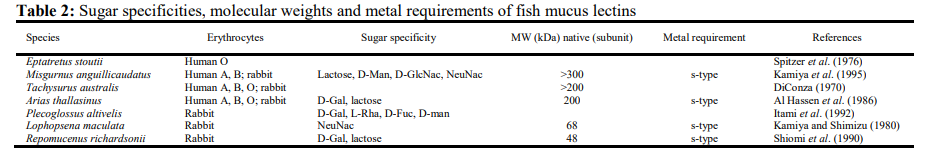
Proteases such as trypsin (serine protease), cathepsin B and L (cysteine proteases), cathepsin D (aspartic protease), and metalloproteases have been identified in fish mucus of Rainbow trout, coho and Atlantic salmon, Japanese eel (Aranishi and Nakane, 1997; Aranishi et al., 1998; Morrissey, 1998; Cho et al., 2000a; Firth et al., 2000; Salles et al., 2007; Subramanian et al., 2007; Fast et al., 2012).
Proteases in skin mucus are also reported for their role in the natural resistance of fish against pathogens (Ingram, 1980). The proteases in skin directly act on a pathogen or may indirectly prevent pathogen invasion by modifying mucus consistency to increase the sloughing of mucus and removing pathogens from the body surface (Aranishi et al., 1998).
Furthermore, proteases are also believed to activate and enhance the production of innate immune components like complement, immunoglobulins or AMPs in Psciene system (Aranishi, 1999).
Several proteases have been characterized in the skin mucus of fishes that display various activities, for e.g. cathepsin D participates in the production of parasin I, a potent antimicrobial peptide from histone H2A in catfish skin mucosa. Cathepsin D inactivates proenzyme procathepsin D and a metalloprotease, which cleaves procathepsin D to generate active cathepsin D.
The activated cathepsin D in turn cleaves the Ser19-Arg20 bond of histone H2A to produce AMPs like parasin I (Cho et al., 2002a). Similarly, expression of a typsin like serine protease in the skin mucus of Atlantic salmon, Salmo salar, in response to the infection with salmon louse, Lepeophtheirus salmonis was demonstrated by Firth et al. (2000).
Aranishi and Nakane (1997) demonstrated the presence of aminopeptidase, cathepsin B and L-like proteases in the epidermal cell layer of Japanese eel (Anguilla japonica) as well as from dorsal surface of European eel (A. anguilla). Cathepsins B and L exhibited high bacteriolytic activity against the fish pathogens Edwardsiella tarda, Flavobacterium columnare and L. Anguillarum (Aranishi, 1999; Aranishi, 2000).
The analysis of skin mucus of five Indian carps demonstrated high protease activity in C. punctata and C. mrigala and low activity in L. rohita and C. catla (Nigam et al., 2012). In another study, the protease activity of epidermal mucus of L. rohita was reported to be highest amongst the three Indian major carps species, i.e. C. mrigala, C. catla and L. rohita (Dash et al., 2014). Proteases isolated from skin mucus of different fish have been summarized in Table 1.
Lectins
The terms agglutinin, phytoagglutinin, hemagglutinin and lectin are interchangeably used to express the naturally existing proteins/glycoproteins with multiple binding sites capable of agglutinating cells or precipitating glycoconjugates (Denis et al., 2003).
interchangeable 교환 할 수 있는
binding site 결합부위
agglutinating 교착성의
precipitate 침전물
glycoconjugate 당결합체
The diversity of these lectins has expanded their definition to include any protein containing a non-catalytic carbohydrate-recognition domain (CRD).
One of the chief events in innate immune defense include the pattern-based recognition of microbial targets as “nonself” by host lectins and related proteins and their subsequent destruction by complement and/or phagocytic cells (Matsushita et al., 2004).
chief 주된
destruction 파괴
These can recognize the non-self cells and enveloped viruses by the means of the carbohydrates present on their surface and then target them for destruction. They may recognize a specific site in sugar or whole sugar or a sequence of sugar and their glycosidic linkages such as glycoproteins and glycolipids or in bacterial polysaccharides on the cell surface glycoconjugates namely (Mercy et al., 1993).
enveloped viruse 외피바이러스
carbohydrates 당질
Enveloped vs. non-enveloped viruses
https://virologyresearchservices.com/2022/05/22/enveloped-vs-non-enveloped-viruses/
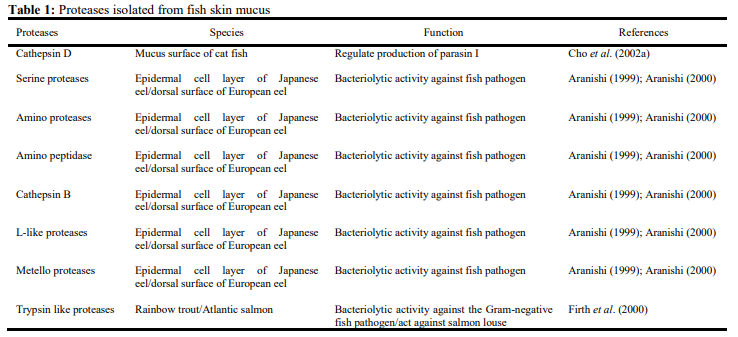
Different types of lectins have been reported in epidermal mucus of fishes. Tsutsui et al. (2011) reported the presence of a new type of skin mucus lectin in cat fish Silurus asotus which displayed Ca+2 dependent mannose binding activity. A mannose binding lectin (MBL) that binds to pathogens was reported in Atlantic cod mucus (Rajan et al., 2011).
Galectins of different forms were also demonstrated to have antibacterial activity (Stowell et al., 2014; Cha et al., 2015). Nattectin, a C-type lectin binding to galactose, was also reported in Atlantic salmon affected by amoebic gill disease (Valdenegro-Vega et al., 2014). Fructose binding lectin was reported in sea bass mucus (Cordero et al., 2015).
Lectins are probably present in the skin mucus of many additional species, because the mucus of many fishes induces hemagglutination, a property typical of lectins (Suzuki, 1995). The sugar specificities, molecular weights and metal requirements of some fish mucus lectins have been summarized in Table 2.
hemagglutination 혈구응집
The Japanese eel has two different types of lectin, AJL-1 and AJL-2. AJL-1 is a galectin, which is characterized by its specific binding to β-galactoside and is homologous to the lectins from Conger eel C. myriaster. In contrast, AJL-2 is a unique lectin, having a highly conserved sequence of C-type lectins but displaying Ca2+ independent activity (Tsutsui et al., 2003). Puff lectin, a mannose specific lectin purified from the skin mucus of the puffer fish, is the third type of lectin (Tsutsui et al., 2003). Puff lectin showed no sequence similarity with any known animal lectins but, surprisingly, shares sequence homology with MBLs of monocotyledonous plants. The fourth type of lectin was found in the pony fish which exhibits homology with rhamnose-binding lectins. Carbohydrates which are specific to this type of lectins are galactose, lactose, fucose, melibiose and rhamnose (Takashima and Hibiya, 1995). A lactose-specific lectin (pentraxin) of molecular mass 25 kDa was purified from the skin mucus of a cartilaginous fish Raja kenojei (Tsutsui et al., 2009). All these observations about lectins in fish mucus suggest that they actively participate in the self-defence system by acting on the external body surface.