3.1 Introduction
The organs and tissues comprising mucosae and mucosal surfaces of teleost fish are both structurally and functionally similar in many respects to their mammalian counterparts including man, despite roughly 450 million years of evolutionary separation.
Teleostean fish were among the first vertebrate animals to display mucosal immune defenses, particularly adaptive immunity within the intestinal tract, as a likely consequence of developing jaws and switching to a predatory lifestyle and the introduction of novel pathogens (Matsunaga, 1998; Matsunaga and Rahman, 1998) as well as to develop the cooperative function between host tissues and immune cells, which first arose within mucosal tissues. This cooperative function became necessary to discriminate between nonpathogens (commensals) from pathogenic organisms (Gomez et al., 2013).
The primary organizational differences of these mucosal immune tissues occur between man and fish due mostly to the environmental niches that each occupy, as the aquatic environment presents unique immunologic challenges to the mucosal tissues of fish through continuous and intimate surface contact of potential pathogens.
It is at this surface interface between the environment and mucosal tissue where first antigenic contact and the immunologic response and processing most often occurs in fish.
Functionally, the response amplitude and efficacy of fish mucosal tissues to immunologic challenges, much like the systemic humoral response, demonstrates a compelling dependency on environmental factors and can be remarkably impacted by local environmental cues such as photoperiod, ambient water temperature, and constituents of water quality such as oxygen saturation, pH, and turbidity (Tort et al., 2004; Niklasson et al., 2011; Uribe et al., 2011).
Population density, social hierarchies and cohort interactions also act as effectors and regulators on response to immunologic challenges (Uribe et al., 2011).
Irrespective of these unique environmental challenges that directly influence outcomes of the mucosal immune response, the first-order constitutive mucosal tissues in teleost fishes that react to immunologic challenges include the gills, integument, and intestine.
Although their physiological roles vary, all of these mucosal tissues share structural similarities at the microanatomic level, notably the presence of an organized epithelial surface with supporting stromal tissues or lamina propria, a vascular supply network, musculature, and resident immune cells. As the intestine is regarded as a conventional or prototypical example of a mucosal tissue, emphasis will be placed on its microanatomic structure and concomitant mucosal immune functions.
3.1.1 Gills
Gills are multifunctional mucosal tissue of fish that are richly vascular and have a massive respiratory surface area much like the alveolar sacs of mammalian lungs; indeed, the basic microanatomic structure of gill filaments and lamellae comparatively resembles pulmonary alveoli turned inside out (Figure 3.1).

Figure 3.1 Hematoxylin and eosin stain (400× magnification). Normal gill filament and lamellar histoarchitecture. In this view, lamellar leaflets extend perpendicularly from the filament body. The filament and lamellae have a monolayer of simple squamous epithelial cells, with basal reserve populations of epithelial cells. Several goblet cells are basally located between lamellar sulci.
At a basic functional level gill filaments and in particular the lamellae are responsible for diffusive and active gas exchange (oxygen and carbon dioxide), active ionoregulation to maintain osmotic and acid–base balance with respect to the environment, localized control of hemodynamics via vasoconstriction and vasorelaxation, and elimination of nitrogenous waste.
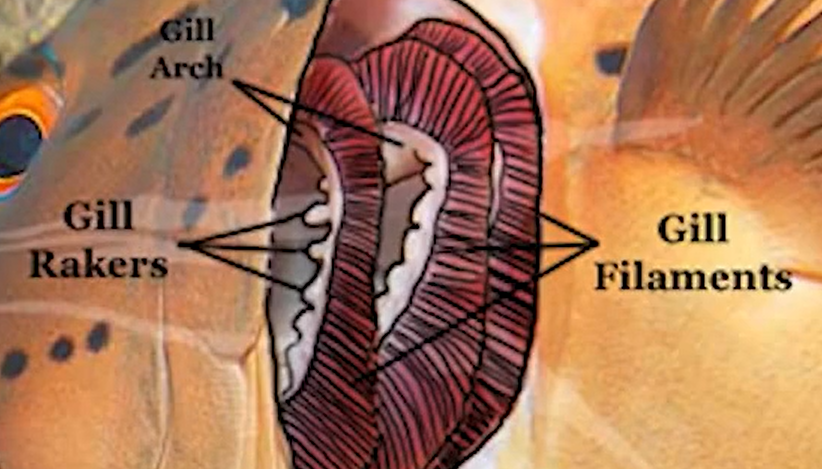
Filaments : where the exchange of oxygen and carbon dioxide actually takes place
Arches : provide structural support for the gills
rakers : appendages that run along the inside edge of the arches, preventing food from passing through the gills
operculum : a hard flap that covers the gills that allows the water pressure to be adjusted in the gills so the fish can breathe without forward movement
Structurally, the microarchitecture of the gill filament is a simple design. Each filament proper is supported internally by a cartilaginous core (or “rod”) and connective stromal or interstitial tissue, percolated by capillaries comprising the two primary respiratory blood vessels (afferent and efferent) and the interlamellar and nutrient blood vessels.
A secondary circulatory system, analogous to mammalian lymphatic vessels, is also present. Arising from the filament body are numerous protruding lamellae or leaflets, which provide the majority of respiratory surface area. These lamellae receive internal support from pillar cells that serve the roles of mechanical support, enable blood circulation, and maintain isolation of the blood plasma from the external environment.
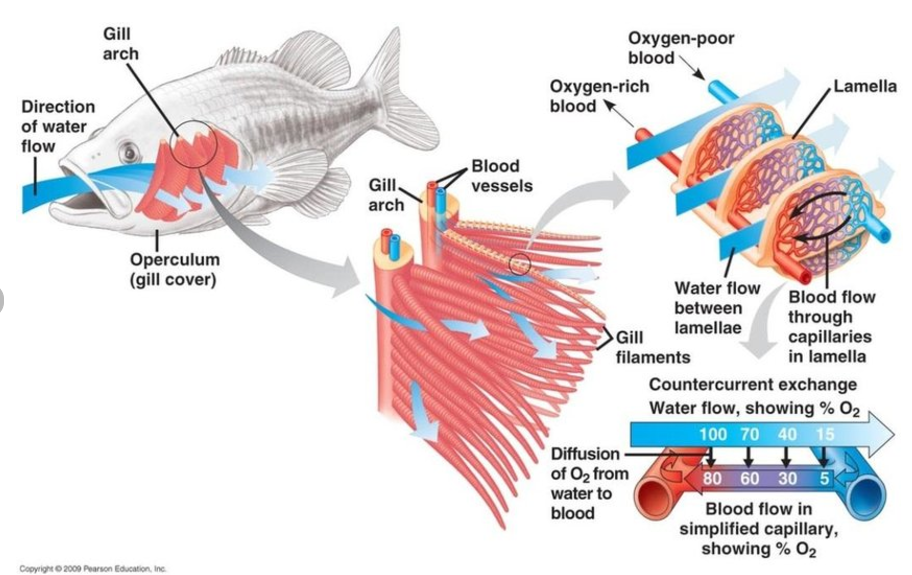
A single epithelial layer of simple squamous cells, often referred to as “pavement cells,” covers the gill filament and lamellae. Microridges present on the surface of these epithelial cells further increases the surface area and provide a platform that enhances adherence of a stable mucous layer.
Interposed among the epithelial cells are a wide variety of specialized cells, including chloride, goblet (mucus-secreting), neuroepithelial, and rodlet cells.
Intracellular goblet cells can occasionally be identified in the squamous epithelial cells, depending on the fish species, the unique positioning of which may allow goblet cells to secrete mucus into the environment and avoid compromising the intercellular junctions of epithelium (Lin et al., 1998).
Taste buds can also be interspersed among the epithelial cells covering the filament, although they are more commonly found within the gill raker epithelium.
The gill rakers, which function to filter particulates and hold prey items, often has abundant lymphocytic aggregates in the subepithelial stromal tissue and may be an anatomic site of primary antigen contact, analogous to the mammalian tonsils.
The surface mucous layer covering the gill filament and lamellar epithelium is an initial physical barrier to pathogen and environmental insults (Dalmo et al., 1997; Magnadottir, 2006) and contains numerous bioactive substances, including enzymes (i.e., lysozyme) and antimicrobial peptides.
Preformed antibodies of the IgM isotype, released from systemic circulation, also reside within the mucous layer. Beyond the physical barrier of mucus, there is a full complement of immune cells, including lymphocytes, plasma cells (antibody-secreting cells), macrophages, neutrophils, and eosinophilic granular cells present in both the intraepithelial spaces of the filament and lamellar squamous epithelium and around the perivascular compartments within filaments.
Lymphocytic populations within the gill tissue are particularly numerous and well characterized, with definitive identification of B cells, plasma cells, and antibodybinding macrophages, and the gills have been recognized as a primary organ for the production of antibodies (DosSantos et al., 2001).
Intraepithelial resident macrophages with capacity to interact with immunoglobulin IgM serve as a frontline defense against soluble antigens, giving a relatively rapid response when pathogens are encountered. The compact interstitium of lamellae can inhibit progressive mobility and interactions with pathogens.
To overcome this, constitutive perivascular plasmacytic cuffs surrounding the interfilament vasculature have an IgM secreting response function to circulating antigens, and antigen trapping occurs within endothelial cells (Davidson et al., 1997; Grove et al., 2006).
The plasma cells and phagocytic endothelial cells likely function in concert to allow an effective, antibody-driven response to pathogens, especially given the constant exposure of the gills to the surrounding aqueous environment. Definable populations of T cells within gill tissue are less certain, although the presence of these cells would be expected, as the expression of interleukins 2, 7, and 15, which drive T cell growth, differentiation, and cytolytic activity, occurs in gill epithelium.
3.2 Integument
The integument of fish is the largest mucosal tissue, respective of surface area, which surrounds the entire animal including fins.
Structural organization and dynamic function of the teleost fish integument varies among different species, from having scales to being scaleless (i.e., catfish) as well as the numbers and types of specialized cells, and can be considered to have the most interspecies differences among the mucosal tissues.
Despite these inherent species differences, the general organization and microanatomic structures are conserved as are the cell types (Figure 3.2).
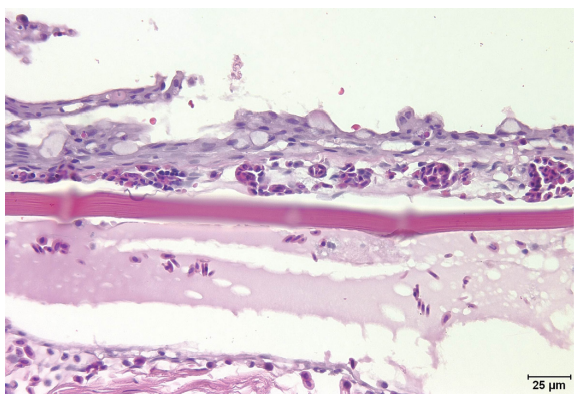
Figure 3.2 Hematoxylin and eosin stain (400× magnification). Normal integument histoarchitecture. In this section, the 5–6 cell thick epidermis is overlying the scale (slightly off-focus in the central photo). Numerous goblet cells punctuate the epidermis and there is multifocal artifactual squamous cell blebbing along the epidermal surface. Wisps of secreted mucus are emanating from some of the goblet cells.
In its simplest organization, fish integument is a comparative precursor to mammalian skin (Rakers et al., 2010) with the notable exceptions of being nonkeratinized (in most species except Asiatic catfish) and containing abundant goblet cells. It is multilayered and has an epidermis with underlying basement membrane, a dermis composed of two discrete tissues, the stratum spongiosum and stratum compactum with an associated subjacent hypodermal space, and a layer of skeletal muscle.
The metabolically active, nonkeratinized epidermis is comprised of several cell types; the most abundant are simple stratified squamous epithelial cells, 5–10 cells in average thickness interspersed with goblet (mucus-secreting) cells.
Similar to the simple squamous epithelial cells of the gill filament and lamellae, integumentary squamous epithelial cells have pronounced microridges that function to provide an adherent surface for mucous secretions and is important in maintaining the mucous gel layer that covers the epithelial surface.
In addition, the epithelial cells have a phagocytic function that may allow them to eliminate not only pathogens but foreign material from the integument, and as they reach near-capacity of internalized material, the cells are sloughed (Åsbakk, 2001; Esteban, 2012).
Goblet cells within the epidermis occur in the outer and intermediate strata of squamous epithelial cells, continuously producing a glycoprotein-rich, viscoelastic mucous secretion. These nonproliferative specialized cells arise from the basal epithelium in early larval life and continuously migrate to their respective epidermal layers, prior to commencing secretory activity (Ottesen and Olafsen, 1997).
Both the number of goblet cells and the biochemical composition of their secretory product vary among fish genera and even between species, depending on local environmental conditions (Fast et al., 2002). The dermis contains predominately fibroblasts intermingling with an ordered collagenous matrix that is punctuated by blood vessels, nerves, pigment cells, and intermixed immune cells.
Scales emanate from vascularized dermal scale pockets and are retained or “anchored” by collagen filaments within the dermis. The epidermal mucous layer contributes significantly to the immune defense against pathogens, as a physical barrier and through elaboration of various preformed peptides, enzymes, and agglutinating lectins (Cho et al., 2002; Nakamura et al., 2004).
Similar to mammals, the fish epidermis has analogous resident dendritic cells, which phagocytize and process antigens (Davidson et al., 1997). Loose aggregates and individual lymphocytes are present throughout the integument, with B cells and plasma cells being the most populous. Secreted antibodies, consisting of IgM and IgT (trout), localize to the epidermal, epithelial, and mucous layers, conferring more specific and additive protection against pathogens (Hatten et al., 2001; Zhao et al., 2008).
Immunoglobulin IgT remains within the integument, while IgM can participate in systemic immune responses, and IgT is compartmentalized to mucosal pathogen interactions (Xu et al., 2013). Detectable antibodies in the integumentary mucus display a lag phenomenon in contrast to serum antibodies when parenteral systemic vaccinations are given, irrespective of the locally available population of plasma cells in the epidermis, which was thought to result from transport of the antibodies through the epidermis to the mucous layer (Rombout et al., 2010), although this observation is more likely due to timing of antibody production in a given systemic response. Bactericidal activity of immunoglobulin IgM sequestered in the epidermal mucus tends to be more reactive against typical fish pathogens than the corresponding humoral activity (Guardiola et al., 2014).
3.3 Intestine
Organization and structural microanatomic features of the fish intestinal mucosa are similar to that of human embryos (Wallace et al., 2005). The intestinal mucosa of teleost fish is thus comparable to mammals in essential structural elements and cell types, with distinct differences that are unique to fish (Figure 3.3).
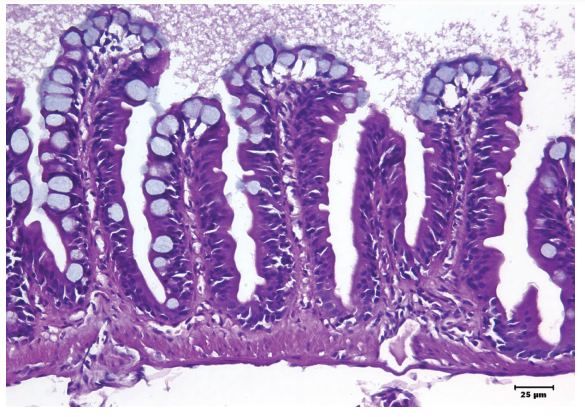
Figure 3.3 Hematoxylin and eosin stain (400× magnification). Normal intestine histoarchitecture. Columnar cells lining the mucosal folds appear pseudostratified in some of the individual folds. Goblet cells are especially prominent at the apices of folds and along lateral aspects. A prominent mucous layer overlies the mucosal folds.
In basic structural organization, the mucosa is comprised of irregular circumferential and longitudinal folds, which may or may not have periodicity and can occur randomly. Transition to longitudinal folds occurs in the posterior intestinal mucosa and mucosal fold height decreases in prominence from anterior to posterior intestinal segments.
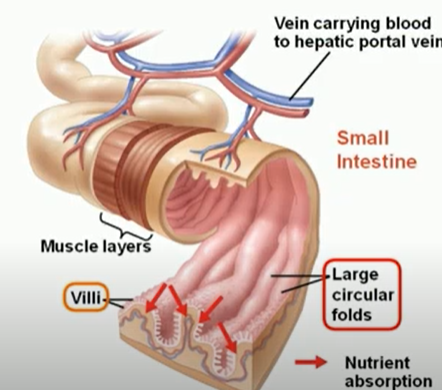
Circular folds : they are coverd with villi. each villus(small finger-like projections), in turn, is covered with microvilli. the microvilli absorb fats and nutrients from the chyme. They slow the passage of the partly digested food along the intestines, and afford an increased surface for absorption.
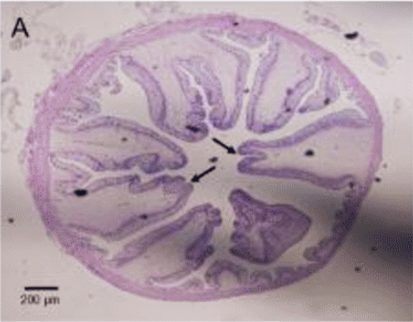
On histologic transverse and longitudinal sections, these folds may often resemble the more conventional mammalian villar microarchitecture, and have on occasion been mistaken for villi. Inter-fold sulci create potential spaces between discrete mucosal folds, and may form pseudocrypt structures that can appear similar to mammalian intestinal crypts.
The basal floor of mucosal pseudocrypts contains actively proliferating epithelial cells similar in fashion to mammalian crypts; however, unlike mammals fish do not have characteristic intestinal mucosal crypts.
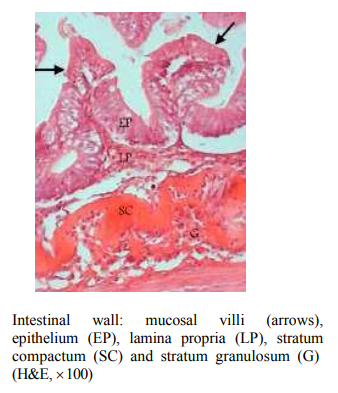
In salmonids there is a unique circumferential band of homogenously dense and supportive collagenous connective tissue immediately subjacent to the lamina propria, called the stratum compactum, which mirrors the same mammalian microanatomic structure in felids.
Variable populations of eosinophilic granule cells intermixed with basophils are frequently located beneath the stratum compactum within the intestinal stromal tissue (Khojasteh et al., 2009; Pirarat et al., 2011), and increases in these inflammatory cells occur during intraluminal parasitic infestation. Additional differences between fish and mammalian intestinal architecture include the lack of a submucosa and muscularis mucosa.
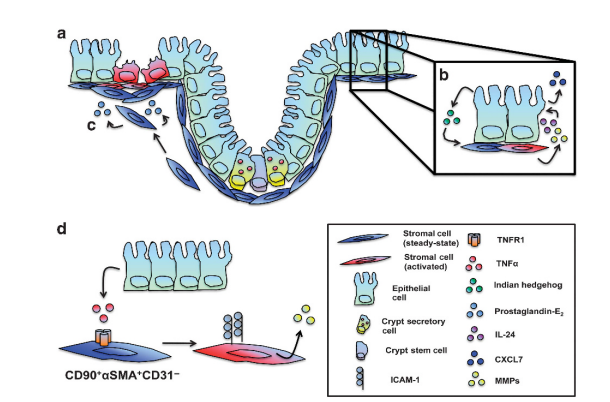
Across most fish species, intestinal mucosal surfaces are covered by a variable surface aqueous boundary layer with an underlying mucous gel layer and lined by a monolayer of simple columnar epithelial cells (enterocytes) that may appear occasionally pseudostratified (Raskovic et al., 2011). Mechanical damage to this mucosal surface layer, from ingestion of hard substances (bone, scales, and gastroliths) and ongoing peristalsis, is thought to enhance local persorption of antigens via the Herbst effect (McLean and Donaldson, 1990).
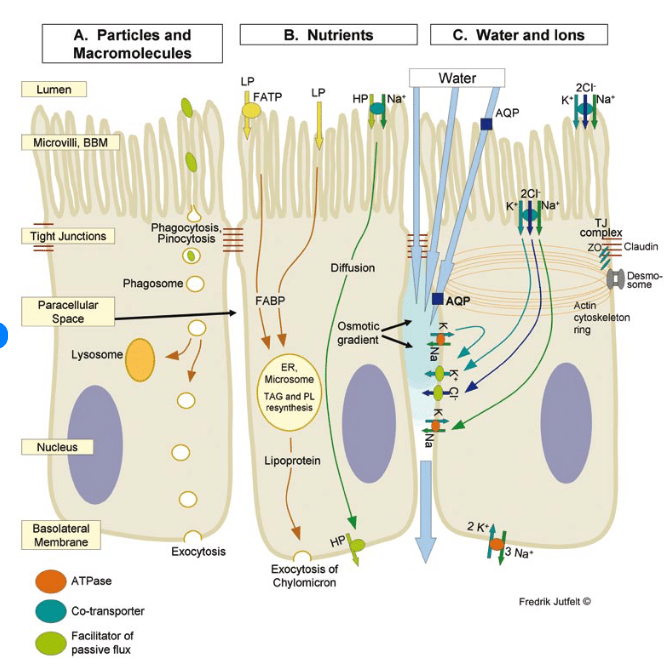
Schematic view of intestinal transepithelial transport in fish. Three enterocytes are shown. Morphological structures in yellow boxes. A. Transepithelial transport of particles and macromolecules starts with phagocytosis or pinocytosis at the brush border membrane (BBM). Phagosomes are transported either to digestive lysosomes or through the cell to the basolateral membrane (BLM) where exocytosis occurs. B. Transepithelial transport of nutrients. Lipophilic molecules (LP) cross the BBM by diffusion over the lipid bilayer of the BBM and through fatty acid transport proteins (FATP). Triacylglycerol (TAG) and phospholipid (PL) resynthesis occur in the endoplasmatic reticulum (ER) before lipoprotein package and export of chylomicrons through exocytosis at the basolateral membrane. Hydrophilic nutrients (HP) such as amino acids and glucose cross the BBM by secondary active transport (sodium co-transport is shown) and cross the BLM by carrier proteins. C. Na+,K+-ATPase activity at the BLM remove intracellular Na+. The NKCC co-transporter carries 2 Cl-, Na+ and K+ across the BBM. Cl-and K+ exit through ion channels across the BLM. The osmotic gradient created in the paracellular space, with the highest osmolality apically drives water diffusion transcellularly and through tight junctions (TJ). Aquaporins (AQP) may increase the BBM and/or BLM water permeability.
Mucus-secreting goblet cells are interposed between enterocytes throughout the juvenile and adult intestinal mucosa, which differs from the larval life stage, where goblet cells are primarily localized to the intermediate segment. The number of goblet cells tends to decrease from the anterior to posterior intestinal mucosa and total numbers of goblet cells are species dependent (Inami et al., 2009).
In larval and juvenile fish, and during inflammation (enteritis), the mucosal epithelium is more permeable to luminal antigens (Dalmo et al., 1997) and response to acute to subacute injury is often denoted by epithelial hyperplasia. In some cases of more chronic enteritis, there may be focal enterocytic separation from the basement membrane, loss of enterocytes, and goblet cell hyperplasia (Ronza et al., 2011), with subsequent metaplasia in severe enteritis. Mucosal epithelium is bolstered by a supporting simple lamina propria, which contains abundant stromal cells, secretory enteroendocrine cells (i.e., serotonin, somatostatin, and enteroglucagon), collagenous support matrices, blood vessels, nerves, and a primitive lymphatic or secondary circulation system that contains intraluminal leukocytes but not erythrocytes (Wardle, 1971; Vogel and Claviez, 1981).
There is some evidence that, at least within trout, intravascular erythrocytes can serve as phagocytic cells for particulate antigens (Jirillo et al., 2007). The existence of a true lymphatic system in fish, comparable to mammals, remains controversial (Press and Evensen, 1999). Putative M-cell analogs have been observed within the posterior intestinal mucosa of zebrafish, as enterocytes in the posterior segment have supranuclear vacuoles that harbor lumen-derived antigens, and these vacuole-containing enterocytes are thought to act as antigen-presenting cells (Wallace et al., 2005). Resident dendritic cells, another class of antigen-presenting immune cells, are present in relatively low abundance within the intestinal mucosa (Gomez et al., 2013). Gut-associated lymphoid tissue (GALT) is widely assumed to be a constant feature among vertebrates (Zapata and Cooper, 1990), although its organization in fish differs with respect to GALT found in mammals and other lower vertebrates, mostly by lacking the welldefined Peyer’s patches (Lin et al., 2005) or the localized lymphocytic aggregates commonly found in birds and reptiles. Fish have referential analogs of mammalian GALT, inclusive of frequent intermixed populations of intraepithelial immune cells as well as loosely aggregated and scattered lymphocytes, eosinophilic granule cells, rodlet cells, neutrophils, and macrophages within the intestinal lamina propria (Reite and Evensen, 2006). Intraproprial lymphocytic aggregates are sometimes colloquially referred to as “cryptopatches” (Matsunaga and Rahman, 1998). The intermediate to posterior mucosal epithelium lining the intestine has been postulated as a critical site for the selection and refinement of GALT-analog lymphocyte populations (Fichtelius et al., 1968). Most intraepithelial lymphocytes tend to reside in the apical mucosal folds while aggregated lymphocyte populations are localized to intestinal pseudocrypts. Lymphocytes are often admixed with other immune cells (Matsunaga, 1998). Although transport of serum immunoglobulin IgM to the intestinal mucosa occurs when pathogens are encountered, a subset of lymphocytes, antibody-producing plasma cells, has been positively identified within the intestine of fish. Locally produced soluble antibodies, such as IgM, are secreted by GALT-analog plasma cells as well as immunoglobulins IgT and IgZ (Flajnik, 2010), which were discovered in trout and zebrafish, respectively. In trout, IgT is preferentially associated with the intestinal mucosa along with compartmentalized IgT-secreting plasma cells (Ye et al., 2013). IgT requires binding to commensal intestinal bacteria in order to interact with potential pathogenic organisms. IgZ is present in the immune organs, including the intestine, of zebrafish and was found on the surface of B cells, indicating that it may have a role as a B cell receptor (Hu et al., 2010). There is a relative paucity of plasma cells secreting immunoglobulin IgD within the intestinal mucosa when compared to other heavy chain isotypes (Ye et al., 2013). Appearance of mucosa-associated and other lymphomyeloid tissues occurs early in embryonic life prior to hatching and sequential development of mature resident neutrophil populations can be observed within 3 days of initial organogenesis, with lymphocyte populations following by the late larval and juvenile life stages (Uribe et al., 2011), but may decrease in overall number as the fish matures to juvenile and adult life stages. Individual fish genera have been employed for uncovering the interrelationship between intestinal mucosal surface interactions with pathogens. Macromolecule and bacterial antigen uptake, via endocytosis, occurs in the posterior intestinal segment of cyprinids with sequential movement from the intestinal lumen into compartmentalized supranuclear vacuoles, which can vary in size depending on the particle size of the ingested material (Nakamura et al., 2004; Inami, 2011). The contained intravacuolar particles are then subsequently to directed to intraepithelial macrophages for phagocytosis and processing (Joosten et al., 1995). Specific changes can occur to supranuclear vacuoles during inflammatory events, including a decrease in size and gradual disappearance that has been noted in cases of soybean meal enteritis, with a corresponding reduction in endocytosis that resolves once the inciting antigen (soy saponins) has been removed from the diet (Penn, 2005; Uran et al., 2008). The hindgut, or posterior intestinal segment, was initially discovered as the preferential site of bacterial antigen uptake by Rombout et al. (1989), where they classified the “second gut segment” in carp as playing a key role in antigen uptake and processing as well as the initiation of a systemic immune response (Rombout et al., 1989). This intestinal segment has been demonstrated to be essential in the elicitation of the both systemic and mucosal immune responses to intraintestinal pathogens. In contrast to cyprinids, gilthead seabream (Sparus aurata) uptake of bacterial antigens did not occur in supranuclear vacuoles but were diffusely distributed in the cytoplasm of enterocytes (Joosten et al., 1995). Transport of bacterial antigens occurred sequentially from the apical to basal intracytoplasmic locations prior to systemic vascular release (Joosten et al., 1995).