INTRODUCTION
The mucosal surfaces of teleosts (bony fishes) are the major interface between fishes and their immediate environment and serve as primary sites of entry for most pathogens. The mucosal surfaces of fishes include the epithelia and associated tissues of the gills, skin, gut, and the reproductive tract.
In mammals, the mucosal system consists of an integrated network of tissues with associated immune cells referred to as the mucosa-associated lymphoid tissue (MALT). It is generally accepted that a comparable system exists in teleosts, although much less is known about its cellular and molecular components and the extent to which they function independently from the systemic immune response.
Although a general understanding of the teleost immune system is emerging, fundamental questions still remain regarding primary lymphoid organ development, the induction, amplification and differentiation of local mucosal immune responses, the production of mucosal antibodies and effector lymphocytes, and immune memory. Answers to these questions will lead to a greater understanding of the evolution of basic immunological mechanisms as well as insights of immediate relevance to applied vaccines and the protection of farm-reared fish from microbial infections.
A number of laboratories have been or are currently engaged in research on mucosal immunity in various fishes, including, carp (Cyprinus carpio) (Rombout et al., 1993), channel catfish (Ictalurus punctatus) (Lobb, 1987; Hebert et al., 2002), rainbow trout (Oncorhynchus mykiss) (Bromage, 2004), Atlantic salmon (Salmo salar) (Lin et al., 1998), sea bass (Dicentrarchus labrax) (Picchietti et al., 1997), zebrafish (Danio rerio) (Danilova and Steiner, 2002) and others because teleosts are a diverse group of fishes, an understanding of the biology of their immune system requires a comparative approach. From the synthesis of research from various laboratories on multiple fish species, a general understanding of mucosal immunity exists and these concepts are presented in each of the chapter sections.
ORGANIZATION OF MUCOSAL TISSUES AND ASSOCIATED IMMUNE CELLS
Gastrointestinal Tract (Fig. 1.1)
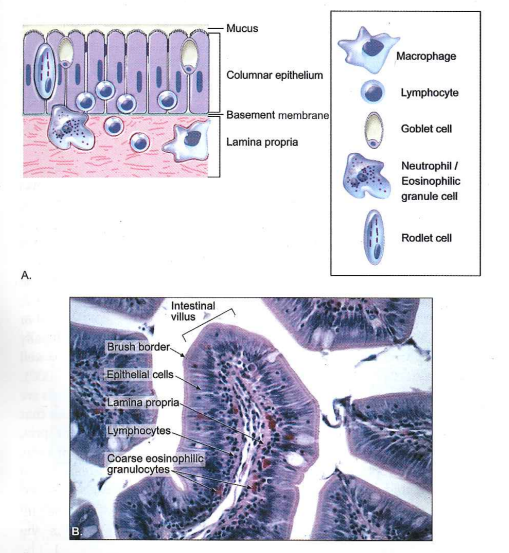
Fig. 1.1 Intestinal epithelium
A. Diagram of the basic anatomical structures of the intestinal epithelium and the identification and location of immune-related associated cells. B. Photomicrograph of the intestinal villus of a channel catfish. Note the mucosal brush border, tall columnar epithelial cells (enterocytes), and supporting lamina propria containing migrating lymphocytes and coarse eosinophilic granulocytes (hematoxylin and eosin [H & E] stain).
The respiratory and digestive systems share the mouth and buccal cavity. The lining of the buccal cavity consists of a stratified mucoid epithelium on a thick basement membrane with a dermis that connects the epithelium to the underlying bone or muscle tissues (Roberts, 2001).
The esophagus has an epithelial lining with large numbers of mucus cells. The stomach varies in size, depending on the species of the fish under study. The gastric mucosa is mucoid with numerous glands in the crypts of the mucosal folds (Roberts, 2001).
Although the intestinal morphology of teleosts varies depending on the species and diet, the intestinal tract has a common basic structure. The intestine is a single tube without the anatomically distinct colon found in mammals (Roberts, 2001).
The rectum has a thicker muscle wall than the intestine and is very mucogenic (Roberts, 2001). The esophagus, stomach, and intestine have four basic layers that vary in composition among and within each of these organs. The innermost layer is the mucosa, which is composed of epithelium, a lamina propria of fibrous connective tissue, and sometimes a muscularis mucosae.
The submucosa, comprised of fibrous connective tissue, lies between the mucosa and the muscularis, which is completely made of muscle. The outer layer of the serosa is composed of fibrous connective tissue covered with a simple squamous mesothelium (Grizzle and Rogers, 1976). The intestinal mucosa is considered to be an immunologically important site in teleosts (Cain et al., 2000).
In carp, the posterior segment of the gut, referred to as the second gut segment, plays a significant role in mucosal immunity (Rombout and van den Berg 1989; Rombout et al., 1989; Rombout et al., 1989) and comprises 20-25% the length of the gut (Rombout et al., 1993; Press and Evensen, 1999). The gut-associated lymphoid tissue of most teleosts, including rainbow trout (McMillan and Secombes, 1997), carp (Rombout et al., 1993), and sea bass (Picchietti et al., 1997) is comprised of cells with lymphoid morphology residing between the gut epithelial cells.
These are predominantly intraepithelial T lymphocytes (Bernard et al., 2006; Huttenhuis et al., 2006), but Ig lymphocytes are also found with the predominant number residing in the lamina propria (Rombout et al., 1993; Danilova and Steiner, 2002; Huttenhuis et al., 2006).
Lymphoid aggregations that resemble the ileal or Peyer's patches in mammals are absent. The GALT of teleosts principally consists of lymphocytes of various sizes, plasma cells, macrophages as well as different types of granulocytes (Du Pasquier and Litman, 2000).
Periodic acid Schiff (PAS) positive cells and eosinophilic granular cells are present, and may serve to modulate immune-hypersensitive responses that occur in the gut. In the intestinal epithelium and lamina propria, macrophages function as scavengers and antigen presenters.
In carp, intestinal macrophages are different from the macrophages isolated from other lymphoid organs in the sense that they adhere poorly to glass and plastic, form clusters with lymphocytes, express antigenic determinants on their outer membranes and bind immunoglobulin (Ig) (Rombout et al., 1986, 1989 a, b, 1993).
The biliary system of the liver begins with intracellular bile canaliculi that anastamose extracellularly to form bile ducts. These fuse into the gall bladder, which directs bile into the intestine through the common bile duct. The gall bladder is lined with transitional epithelium. Hematopoietic tissue with melanomacrophage centers is associated with larger blood vessels of the liver (Roberts, 2001).
Skin (Fig. 1.2)
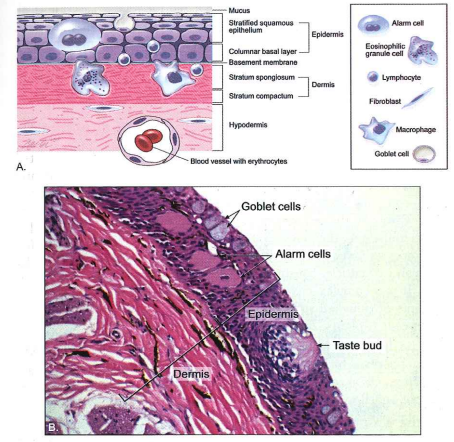
Fig. 1.2 Skin epithelium
A. Diagram of the basic anatomical structures of the skin and the identification and location of immune-related associated cells. B. Photomicrograph of channel catfish skin (sensory barbel) (H & E stain).
The skin of fishes provides protection against physical, chemical and biological damage. It consists of two anatomical layers, the epidermis and dermis. The thickness of the stratified epithelium of the epidermis varies, depending on the area of the body, age, sex, maturation and environmental stresses (Grizzle and Rogers, 1976; Yasutake and Wales, 1983). On average, it has a thickness of 10-12 cells.
Cells in the basal columnar layer of the epidermis, referred to as the stratum germinativum, replicate and move toward the surface of the fish. This basal layer lies immediately above a basement membrane. At least six types of cells have been described in the epidermis of teleosts, including filament-containing malpighian cells (keratinocytes), mucus cells, chemosensory cells, club cells (alarm substance cells), granule cells and chloride cells (Grizzle and Rogers, 1976; Yasutake and Wales, 1983; see for review zaccone et al. 2001).
The malpighian cells are the most abundant in the epithelium. These cells are rounded in shape with bundles of fibers and mitochondria around a generally ovoid nucleus (Roberts, 2001). At the epithelial surface, keratinocytes become more flattened and their cytoplasm consisting predominantly of oblong vesicles, degenerating mitochondria and denser bundles of fibers. The outermost layer of cells is not keratinised.
The surfaces of the outermost cells have convoluted microridges of an unknown function that possibly assist in holding mucous secretions to the skin. Mucus cells begin differentiating in the stratum germinativum and migrate to the surface of the skin where they release their contents. Packets of mucus are bound by membranes and progressively fill the cell as they move toward the surface. At the surface of the epithelium, the mucus cell (a holocrine gland) moves between the keratinocytes and discharges its contents. The epidermis is covered by a glycocalyx or cuticle, consisting of a thin (1.0 µm) mucopolysaccharide layer. It is a complex mixture of molecules derived primarily from the contents of sloughed surface epithelial cells and mucus secreted from goblet cells (Roberts, 2001).
The deeper layers of the epidermis contain alarm substance cells and melanophores, which do not reach the surface. The contents of alarm substance cells are only released when the epidermis is physically damaged (Grizzle and Rogers, 1976), Capillaries extend into the epidermis from dermal papilli, and come within 10 cell layers of the surface (Lobb, 1987). The dermis is composed of two layers. The upper layer, referred to as the stratum spongiosum, consists of a loose network of collagen and reticulum fibers and is contiguous with the epidermal basement membrane that lies just above it. It contains chromatophores, mast cells and the cells of the scale beds. The lower layer, the stratum compactum, is a dense matrix of collagen that provides the structural strength to the skin. The hypodermis, lying beneath the dermis, is composed of loose connective tissue. It is more vascular than the overlying dermis. Melanophores occur in the hypodermis, dermis and sometimes in the epidermis. No organised lymphoid germinal centers have been found in the skin (Flajnik, 1998), although cells with the morphology of lymphocytes can be detected by light microscopy in stained tissue sections of channel catfish skin (Lobb, 1987).
These cells occur throughout the epidermis and are located predominantly near the stratum germinativum at the junction of the epidermis and dermis (Lobb, 1987). Antigen-specific and total antibody secreting cells (ASC) have been isolated from the skin of channel catfish and detected by ELISPOT (Zhao et al., 2007). B cells isolated from the skin of channel catfish can be stimulated with LPS to replicate and secrete antibody in vitro, a response that, in turn, is abrogated by the addition of hydroxyurea to the culture medium (Zhao et al., 2007). Macrophages are also present in the skin (Roberts, 2001).
Gills (Fig. 1.3)
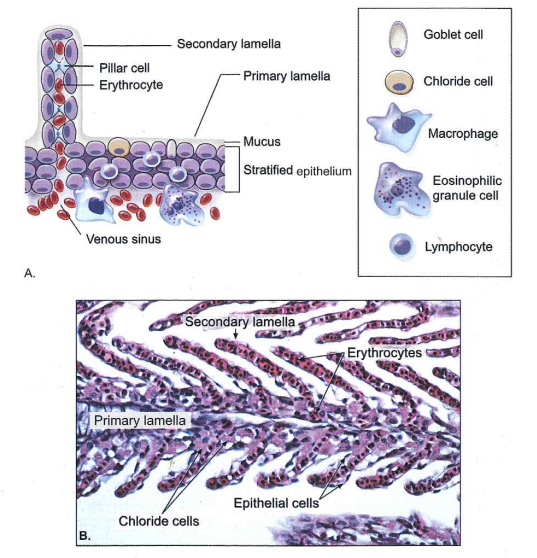
Fig. 1.3 Gill epithelium
A. Diagram of the basic anatomical structures of the gill epithelium and the identification and location of associated immune-related cells. B. Photomicrograph of fish gill. Note the capillaries with erythrocytes in secondary lamellae and chloride cells concentrated in lamellar troughs (H & E stain).
The gills consist of gill arches, gill filaments (primary lamellae), and gill lamellae (secondary lamellae). Two rows of filaments are present on each arch and the secondary lamellae branch out perpendicularly from the filaments (Grizzle and Rogers, 1976; Yasutake and Wales, 1983). The gill arches and filaments are supported by a branching system of cartilaginous rods. A stratified squamous epithelium covers both the gill filament and the gill lamellae. The lamellae provide the actual respiratory surfaces.
Each lamella comprises a network of interconnected spaces that are separated and supported by pillar cells. Blood enters the lamellae from the afferent arterioles of the filaments and exits into the efferent arteriole. The lamellar intercellular spaces through which blood flows are lined with endothelial cells. A basement membrane lies over the endothelial cells and pillar cells, which form supportive 'flanges' around the intra-lamellar spaces (Grizzle and Rogers, 1976). The stratified epithelium itself is only one to two cells thick in order to allow gas exchange, a degree of thinness that makes the tissue vulnerable to invasion by pathogens.
Different cell types are associated with the gill epithelium. Chloride cells function in the transport of Cl and other ions across the epithelium. These cells are more spherical than those that surround them in the epithelium; they project somewhat above the surface (Yasutake and Wales, 1983), and their cytoplasm is more eosinophilic (in hematoxylin and eosin stained sections) than is the case with other epithelial cells. Chloride cells are abundant in the gill filament epithelium between lamellae (Grizzle and Rogers, 1976). Mucus cells are abundant in the lamellar epithelium, and appear under light microscopy as mucus-filled domes or vacuolated cells (Yasutake and Wales, 1983). Goblet cells are most abundant on the margins near arterioles. Alarm substance cells are absent in gill epithelia (Grizzle and Rogers, 1976). Although the surface of the gill lamellar epithelium is irregular, it does not have the distinctive microridges seen on the surface of the skin epidermis (Roberts, 2001).
Nevertheless, these irregularities are sufficient to aid in attachment of mucus, which in addition to its role in reducing invasion of microorganisms, also serves to regulate the transfusion of gases, ions, and water across the epithelial membrane (Roberts, 2001). Similar to the situation that exists in skin, there is no evidence to indicate the existence of organised aggregations of lymphoid tissue in the gills. Nevertheless, there have been a number of studies to show the functional immunological activity in gills as well as gill-associated leucocytes and lymphocytes (Goldes et al., 1986; Powell et al., 1990; Lumsden et al., 1995; Davidson et al., 1997; Lin et al., 1998; Rombout et al., 1998; Dos Santos et al., 2001 a,b).
Considerable numbers of lymphocytes, ASC, and macrophages were found to reside in the gill tissue of Atlantic salmon and dab (Lin et al., 1998). In leucocyte suspensions from carp gill (as in skin), Rombout et al. (1998) found an abundant population of intraepithelial lymphocytes (IEL) that reacted with a monoclonal antibody (mAb WCL38), which is specific for IEL T cells in the carp intestine. In gill IEL leucocyte suspensions, WCL38+ cells comprised the major population of lymphoid cells.
Lymphocytes with surface immunoglobulin (i.e., B cells) were a minor component of these cell populations. In cryosections, many of the WCL38+ cells were detected at the base of the gill lamellae. Immunogold labeling showed that the WCL38+ cells had the ultrastructure of lymphoid cells, although two morphologically different cell types were found: small lymphocytes with a high nucleus/cytoplasm ration, and larger granular lymphocytes with a lower nucleus cytoplasm ration and a variable amount of electron-dense, lysosome-like material.
Ontogeny of Mucosal Lymphocytes and Immune-associated Cells
Lymphocytes and other cells (such as macrophages) that function in acquired immune responses of teleosts are present in gut-associated immune tissues and other mucosal tissues and most likely evolved in these sites early in the development of the vertebrate adaptive immune response (Matsunaga, 1998; Matsunaga and Rahman, 1998; Cheroutre, 2004).
In present-day teleosts, however, the ontogeny of mucosal lymphocytes has not been resolved and the extent to which they develop and remain resident in mucosal tissues or migrate to and from primary and secondary lymphoid organs, such as the head kidney and spleen remains to be determined. The mammalian gut can function as a primary lymphoid organ and intraepithelial lymphocytes (IEL) develop at this site (Lefrancois and Puddington, 1995) and, as indicated above, it is likely that the early adaptive immune system of vertebrates also evolved in gut epithelium (and possibly skin and gill epithelia as well) (Matsunaga and Rahman, 1998; Cheroutre, 2004). With the evolving adaptive immune system, however, the thymus acquired the mechanisms of lymphocyte maturation and selection and subsumed this function from mucosal sites. Thus, gut mucosal tissues were eventually relieved by the thymus of the responsibility to educate the developing IEL regarding self and non-self (Cheroutre, 2004).
Immune mechanisms of mucosal surfaces have been extensively studied in higher vertebrates and the roles of specific T and B cells located in epithelia are elucidated (Cheroutre, 2004). For example, in mice, it has been shown that αβ T cells localised in epithelia migrate from the GALT and peripheral lymphoid tissues following antigen stimulation (Kim et al., 1998). In this process, specialised cells in the follicle-associated epithelium of the gut, referred to as M cells, sample the lumen of the gut and transport antigens to the subepithelial tissues and GALT (Neutra et al., 1996).
Local dendritic cells then process these antigens and further distribute them to peripheral lymphoid tissues in which resident naïve αβ T cells become activated and proliferate. These antigen-specific, differentiated T cells then migrate to the gut where they seed the epithelium as effector and memory cells. There also are specialised IEL in the mammalian gut that develop via an extrathymic pathway (Lefrancois and Puddington, 1995).
These IELs mostly consist of γδ T cells with a oligoclonal TCR repertoire (Regnault et al., 1994; Cheroutre, 2004; Bernard et al., 2006). The mechanisms responsible for the limited repertoire is unknown, but is believed to be the result of selection during lymphocyte development in the gut (Takimoto et al., 1992), a process involving resident microflora (Helgeland et al., 2004). It has been suggested that extrathymic development of T cells occurs in teleosts as well, at least in carp, a species in which the first studies of mucosal lymphocyte ontogeny have been systematically carried out (Huttenhuis et al., 2006),
These studies showed that IELs develop in embryonic gut epithelia before the development of the thymus. In addition, the expression of rag-1 in intestinal tissues was seen to occur concurrently with the early appearance of these intestinal IELs. However, there may be species-specific differences among teleosts regarding IEL ontogeny. A recent immunoscope-based analysis (Pannetier et al., 1995) of the VBJB spectratypes of IEL and systemic T cell receptors (TCR) in trout showed that intraepithelial T lymphocytes isolated from the gut of naïve fish have similar TCR repertoires to T cells found in the blood and spleen (Bernard et al., 2006). While this finding does not preclude an extrathymic development pathway for IEL or a subpopulation of IEL not surveyed in this study, it suggests (at least in trout) that αβ IEL correspond to random samples of systemic αβ T cells (Bernard et al., 2006).
The predominant population of IEL in the mammalian gut consists of γδ T cells, which are suggested to have evolved before αβ T cells in the development of adaptive immunity. Although the genes encoding the γδ TCR have been identified in the Japanese flounder (Paralichthys olivaceus) (Nam et al., 2003), the extent to which teleost lymphocytes equivalent to the γδ T cells exist in populations of IEL is still not known. The development of reagents such as monoclonal antibodies to identify characteristic cell surface receptors and ancillary proteins in teleosts will be necessary to answer this question (Miller et al., 1998). B cells occur in mucosal tissues, but current evidence suggests that they develop in the primary lymphoid tissues of the head kidney. In zebrafish, B cells are first found to appear in the embryonic pancreas, and then the head kidney (Danilova and Steiner, 2002). In carp, B cells first appear in the head kidney and spleen of embryos, and later in mucosal organs and the thymus, but Ig lymphocytes are never abundant in the thymus and intestine (Huttenhuis et al., 2006).
MUCOSAL INNATE IMMUNITY
The mucosal surfaces of the skin, gills and intestine are constantly exposed to environmental pathogens; yet, under normal circumstances they remain free from infection and life-threatening lesions. Epithelia also heal quickly following mechanical or chemical injury. Resistance to infection and recovery from traumatic insult is facilitated by innate non-specific immunity that consists of a plethora of constitutively expressed elements as well as induced components of the inflammatory response. The physical factors of innate immunity consist of the membrane-anchored surface mucus barrier (glycocalyx) and the contiguous underlying epithelial cells with their tight junctions. The components of innate immunity can be generally classified as either cellular or humoral effectors.
Cellular Components of Mucosal Innate Immunity
Teleosts have interacting leukocyte subpopulations that mediate both innate and adaptive immune responses (Miller et al., 1998). Cell populations involved in the innate immune response include phagocytic cells (macrophages and neutrophils), non-phagocytic cells (natural killer (NK) cells and non-specific cytotoxic (NCC) cells), and other cells (mast cells/eosinophilic granule cells and rodlet cells).
Mast cells/eosinophilic granular cells have structural and functional properties similar to those of mammalian mast cells (Reite, 1997), and store a number of inflammatory and anti-microbial compounds, including phospholipids, alkaline phosphatase, peroxidase and lysozyme (Silphaduang et al., 2006).
Rodlet cells occur in blood and epithelia of a large number of teleost species (Reite, 1997, 2005) and have a characteristic morphology with cytoplasmic club-like crystalline inclusions that are released at epithelial, mesothelial and endothelial surfaces.
Although there is still some question as to the origin and function of these cells, most recent studies interpret rodlet cells to be elements of the host defense system, appearing in association with insult from various stressors including parasites, neoplasia, viral infections, and general tissue damage (Reite, 1997, 2005; Manera and Dezfuli, 2004; Bielek, 2005; Reite and Evensen, 2006; Silphaduang et al., 2006).
The innate cell inflammatory response of teleosts is usually biphasic, beginning with an influx of neutrophils followed by the arrival of monocytes and macrophages (Sharp et al., 1991; Neuman et al., 2001). Neutrophils originate from the head kidney, while macrophages originate from blood-derived monocytes that migrate to the relevant tissues following inflammatory insult.
Monocytes develop from hematopoietic stem cells in the head kidney and/or the spleen. In addition to the phagocytic cells that extravaginate and migrate to tissues during inflammation, mucosal tissues also have resident macrophages that are involved in the ingestion of antigens and antigen presentation and are postulated to play an important role in both innate and acquired immune responses (Huttenhuis et al., 2006).
Gastrointestinal Tract: The gastrointestinal tract of teleosts contains intraepithelial macrophages as well as neutrophils and mast cells/ eosinophilic granular cells (MC/ECG) located in the lamina propria (Georgopoulou and Vernier, 1986; Vallejo et al., 1989; Rombout et al., 1989, 1993b; Davidson et al., 1991; Powell et al., 1991; Dorin et al., 1993; Sveinbjornsson et al., 1996; Hebert et al., 2002; Leknes, 2002; Grove et al., 2006).
In experiments carried out in platy (Xiphophorus maculatus), horse- spleen ferritin injected into the coelomic cavity was taken up by macrophages located primarily in the lamina propria of the gut (Leknes, 2002). A MC/ECG submucosal layer is well developed in salmonids.
MCs/ECGs can move from the submucosal layers of the intestine into the villi or mucosa, as also in certain allergic and bacterial infections marked degranulation of these cells occurs. Experimental intracoelomic injection of extracellular products from culture supernatants of the bacterium Aeromonas salmonicida elicited vasodilatation of blood vessels in the lamina propria of the gut with concomitant dissemination and degranulation of the MC/ECG cells (Ellis et al., 1981).
Rodlet cells often occur associated with the presence of adult parasitic trematodes or cestodes in the intestine and with encysted helminth larvae in the intestine or its adjacent tissues (Reite, 1997; Dezfuli et al., 1998; Bielek, 2005).
A significant number of neutrophils (> 64% of leukocyte cells isolated from collagenase-digested intestine) appear to reside in the gut of healthy juvenile channel catfish, suggesting that innate immunity plays an important role in host defense in this species (Hebert et al., 2002). Likewise, in gilthead seabream (Sparus aurata), acidophilic granulocytes (considered equivalent to neutrophils in this species) occur principally dispersed in the lamina propria of the mucosa in the posterior intestine (Mulero et al., 2007).
It is hypothesised that these cells play an important role in innate immunity and immune surveillance and studies have shown that the administration of probiotics to gilthead seabream elicits an increased number of these cells in the gut (Picchietti et al., 2007). Ig lymphoid cells are diffusely distributed within the epithelia of the gut. Although this population consists mainly of intra-epithelial lymphocytes (IEL, primarily putative T cells), NK cells are postulated to occur here as well (Rombout et al., 1993).
Isolation of cytotoxic IELs from the intestine of rainbow trout have been isolated and functionally characterised with regard to non-specific killing of target cells. These cells did not contain cytotoxic granules analogous to those seen in mammalian NK cells, suggesting an alternative mechanism for cell killing (McMillan and Secombes, 1997). Skin: Macrophages, neutrophils and other granulocytes such as MC/ ECG appear in the deeper layers of the epidermis, particularly in response to inflammatory events such as parasitic infection (Cross and Matthews, 1993; Buchmann, 1999; Reite and Evensen, 2006). In rainbow trout and channel catfish, migratory macrophages and lymphocytes are present in the skin (Lobb, 1987; Peleteiro and Richards, 1990).
Activation of fish leucocytes in vitro elicits the production of leukotriene B4 which, in turn, induces the migration of neutrophils (Hunt and Rowley, 1986).
Teleost macrophages and neutrophils secrete interleukin 1, which affects other macrophages (Secombes and Fletcher, 1992). These signaling molecules are likely to play a role in the induction and activation of the cellular innate immune response in the skin.
Langerhans cells are dendritic antigen-trapping cells found in the human skin and have the ability to process and present antigen to lymphocytes (Koch et al., 2006). These cells have a typical granular cytoplasm and defined cell surface determinants. Reports of resident antigen-trapping phagocytic cells in teleost skin are rare (Peleteiro and Richards, 1990), with only one reference to epidermal cells with membrane folding that resembles the Birbeck's granules typical of human Langerhans cells (Mittal et al., 1980).
Although phagocytic cells with the typical morphology of Langerhans cells apparently do not occur in the epidermis of fish, this does not preclude the possibility that dermal macrophages, which migrate across the basement membrane into the epithelium, do trap and process antigen. Indeed, phagocytic cells that share cell surface determinants with Langerhans cells (referred to as indeterminate or agranular dendritic cells) exist in human epithelia (Rowden et al., 1979), and it is postulated that these are monocyte- derived dermal macrophages that migrate into the epidermis and develop into Langerhans cells (expression of surface determinants and formation of Birbeck's granules) under the influence of chemokine gradients and a particular epithelial micro-environment (Koch et al., 2006).
It has been hypothesised that the migration of macrophages into the epidermis of fish could be the equivalent of these non-differentiated Langerhans precursor cells seen in human skin (Peleteiro and Richards, 1990). NK or NCC cells have not been reported in the skin, but it is possible that activated cells recruited from the head kidney into the peripheral blood could end up in this peripheral site (Graves et al., 1985).
Gills: In addition to epithelial cells, mucus-secreting goblet cells and chloride cells described earlier, various types of leukocytes have been isolated from the gills of teleosts. Macrophages, eosinophilic granular cells (EGC) and neutrophils have been isolated and characterised in perfused gill tissue from Atlantic salmon and dab (Limanda limanda) (Lin et al., 1998).
In experiments carried out in platy (Xiphophorus maculates), horse- spleen ferritin injected intracoelomically was taken up by macrophages located within the gill filament, but not the gill lamellae (Leknes, 2002). Thus, while the main functions of gill phagocytes are presumably to capture foreign substances and kill infectious agents that gain entry from the water, these cells also apparently participate in the clearance of foreign substances from the blood (Leknes, 2002). Although resident dendritic cells have not been described in gill epithelia, gill macrophages most likely process and present antigenic material to lymphocytes to initiate a specific, acquired immune response (Davidson et al., 1997; Lin et al., 1998).
Humoral Components of Mucosal Innate Immunity
The mucus coating of fish skin, gills and gut epithelia is a complex mixture comprising molecules secreted by goblet cells and cellular contents released from effete surface epithelial cells. The major component of mucus is mucin, which is composed mainly of glycoproteins. Also present are lysozyme, proteolytic enzymes, and C-reactive proteins (Ingram, 1980; Fletcher, 1981).
Mucus acts as both a physical and chemical barrier to microbial invasion and environmental insult.
Non-immunoglobulin humoral defense factors in fish have been classified into four general categories based on their effects on invading pathogens: (1) microbial growth inhibitory substances, (2) enzyme inhibitors, (3) lytic agents (lysins), and (4) agglutinins/precipitins (Alexander and Ingram, 1992).
Various antimicrobial compounds in these categories including trypsin, lysozyme, lectins, complement, and other lytic factors are present in mucus and mucosal tissues where they serve to prevent adherence and colonisation of pathogenic microorganisms (Alexander and Ingram, 1992; Dalmo et al., 1997). These factors are described below with specific indications of their roles in mucosal innate immunity, if known to occur.
Microbial Growth Inhibitory Substances: The microbial growth inhibitors transferrin, caeruloplasmin, metallothionein, and interferon-are all present in fish tissues (Alexander and Ingram, 1992).
Transferrin is an acute phase protein that is elicited during inflammation to remove iron from damaged tissues, and activate macrophages (Magnadottir, 2006). It is expressed constitutively in liver cells.
Lactoferrin, a protein related to transferrin, occurs in mucus secretions of mammals, but has not been reported in fish mucus or epithelial cells (Alexander and Ingram, 1992).
Interferons (IFN) are secreted proteins that activate cells to an anti-virus state, inducing the expression of Mx and other antiviral proteins (Leong et al., 1998; Robertson, 2006). Type I IFN α and β and type II IFN γ have been detected or deduced in a number of different fish species (Graham and Secombes, 1990; Alexander and Ingram, 1992; Robertson, 2006). IFN γ produced in NK cells modulates innate immune responses; but as indicated earlier, there have been no studies to indicate whether or not NK cells are found in mucosal tissues.
Enzyme Inhibitors: The basic function of enzyme inhibitors is to maintain homeostasis of blood and other body fluids through the regulation of enzyme activities including those involved in the functions of complement activation and coagulation (Alexander and Ingram, 1992). Following invasion by pathogens, destructive enzymes are actively secreted into tissues by parasites and passively released from damaged host cells including neutrophils and macrophages that have migrated to the site of infection.These released proteases require inactivation to prevent and reduce secondary tissue destruction.
A plethora of proteinase inhibitors (serine, cysteine-, and metalloproteinases) have been isolated and characterised in mammals, but few have been described in fishes. The most widely studied in fishes is x2 macroglobulin, which has broad inhibitory effect through encapsulation of protease molecules (Armstrong and Quigley, 1999; Magnadottir, 2006). The extent to which enzyme inhibitors function at the mucosal surfaces is currently unknown.
Lytic Agents: The lytic components of humoral innate immunity are enzymes that exist as either single molecular entities, such as lysozyme, or a cascade of component enzymes as occurs in the complement system. Lysozyme has been found in tissues and secretions of fish including the gut, cutaneous mucus and gills (Alexander and Ingram, 1992; Magnadottir, 2006), where it is produced by macrophages, neutrophils, and eosinophilic granule cells (Murray and Fletcher, 1976).
Lysozyme attacks structures containing β1-4 linked N-acetylmuramamine and N-acetylglucosamine, (the peptidoglycan components of bacterial cell walls), as well as chiton, a component of fungal cells and is, thus, both antibacterial and antifungal. It also functions as an opsonin with subsequent activation of complement and phagocytes (Magnadottir, 2006). The amount of enzyme varies among tissues and species of fish (Alexander and Ingram, 1992). Lysozyme has been described in the cutaneous mucus of a number of fish species, including carp and channel catfish.
The teleost complement system consists of more than 35 soluble plasma proteins that play roles in both innate and acquired immunity (Boshra et al., 2006). Complement activation products initiate or are involved in the innate immune functions of phagocytosis and cytolysis of pathogens, solubilisation of immune complexes, and inflammation (Boshra et al., 2006). There are only a few experimental studies that address the extent to which the components and functions of complement occur in mucosal tissues and secretions.
A study showing that the parasitic monogenetic trematode Gyrodactylus salaris was killed following incubation in cutaneous mucus of Atlantic salmon suggests that components of the complement system are involved in the innate immune responses of the skin. In this study, mucus activity was approximately one twentieth of that found in serum.
Activity (in serum) was not dependent on the immune status of the fish and opsonisation of parasites with antibodies did not enhance killing, suggesting that complement was activated by the alternative pathway (Harris et al., 1998) or by the lectin pathway (Buchmann, 1998, 1999). Transcripts of complement factors C3 (rainbow trout) and C7, P (FP), Bf/C2A, C4, and D (FD) (carp) were detected in the skin following infection with the ciliated protozoan parasite Ichthyophthirius multiflüs (Sigh et al., 2004; Gonzalez et al., 2007 a, b).
These studies also suggest that parasite infection elicits expression of a subset of extrahepatic complement genes in the skin. It is postulated that the proteins are produced in macrophages (Buchmann, 1999). Cutaneous mucus of Japanese eels (Anguilla japonica) contains a locally produced hemolysin that could have a non-specific protective role, although this has not been determined (Alexander and Ingram, 1992).
Trypsin has been found in mucus and mucus-secreting cell layers of the skin, gill lamellae, and anterior intestine of Atlantic salmon and rainbow trout, where it is hypothesised to play a role in non-specific immunity against microbial invasion at these surfaces (Hjelmeland et al., 1983; Braun et al., 1990). It should be noted that the presence of active trypsin at these surfaces suggests that enzyme inhibitors are not present.
Agglutinins: Agglutinins are agglutinating factors (non- immunoglobulin) produced in the absence of defined antigenic stimuli (Ingram, 1980). These carbohydrate-binding proteins elicit opsonisation, phagocytosis and activation of the complement system (Buchmann, 1999). Mucosal agglutinins and precipitins consist primarily of lectins such as C-type lectins and pentraxins. In the presence of Ca⁺, C-type lectins bind mannose, N-acetylglucosamine and fucose leading to opsonisation, phagocytosis and activation of the complement system (Magnadottir, 2006).
Pentraxins, which include C-reactive proteins, are commonly associated with the acute phase inflammatory response and take part in innate immunity by activating complement pathways. A hemagglutinin is found in the cutaneous mucus of Japanese eels but the extent to which it is involved in innate immunity is not known (Magnadottir, 2006). Lectins found in cutaneous mucus appear to play a role in the innate immune response against parasites of the skin, such as the ciliate I. multifiliis, and the trematode Gyrodactylus (Yano, 1996; Buchmann, 1999; Buchmann et al., 2001; Xu et al., 2001). Nevertheless, the roles of mucus lectins remain unresolved in many cases and it is possible that they could work independently or in cooperation with other biologically active molecules (Alexander and Ingram, 1992).
Natural Antibodies: Although antibodies (immunoglobulins) are generally considered to be the primary effector mechanism of the humoral acquired immune response, natural antibodies are also considered to be components of the innate immune system.
There are different sources of natural antibodies including: adoptive transfer, environmental antigen exposure, and production by gene rearrangement without specific antigen stimulation (Sinyakov et al., 2002; Magnadottir, 2006). Natural antibodies have increasingly been shown to play a role in mammalian immunity and their occurrence and function in immunity in fishes also has been well documented (Sinyakov et al., 2002; Magnadottir, 2006).
The fact that specific antibodies are produced locally in mucosal tissues would suggest that natural antibodies also could occur in these sites, although no systematic studies have been done to determine this. In vaccine studies with channel catfish, however, a relatively small but consistent number of antibody secreting cells (plasma cells) that produce antibody against the major surface antigen of I. multifiliis have been detected in skin epithelia of naïve fish (Dickerson, unpubl. data). These could be natural antibodies. Given the importance of the surface mucosa as a first line of defense against pathogens, it seems logical to expect that natural antibodies would occur in these sites. More research is necessary in this area.
Antimicrobial Peptides: Low molecular weight antibacterial peptides in vertebrates are usually associated with peripheral blood leucocytes or mucosal surfaces (Bevins, 1994; Cole et al., 1997; Smith et al., 2000; Silphaduang et al., 2006).
They have a number of useful characteristics for innate immune responses, namely, broad spectra of activity against microorganisms, low toxicity for host cells, ease of synthesis, and rapid diffusion rates (Smith et al., 2000).
Antimicrobial peptides have been described in the skin from a number of different fish species, including rainbow trout, where mucus extracts were shown to have muramidase and non-muramidase lytic activity against selected bacteria (Smith et al., 2000; Ellis, 2001). The peptide piscidin has recently been found in a wide range of teleost species and is produced in gill, skin, stomach and intestinal epithelia. Piscidin is produced in MC/eosinophilic cells and rodlet cells (Cole et al., 1997; Silphaduang et al., 2006). The presence of piscidins in eosinophilic cells, which occur in epithelial tissues, suggests that they play an important function in innate defenses in these tissues (Silphaduang et al., 2006).
'The biology of teleost mucosal immunit' 카테고리의 다른 글
| The biology of teleost mucosal immunity- MUCOSAL ADAPTIVE IMMUNITY (0) | 2025.01.11 |
|---|