MUCOSAL ADAPTIVE IMMUNITY
The adaptive mucosal immune response of teleosts, which is postulated to have appeared early in the evolution of acquired immunity, plays an important role in protection against infection. Fishes are the earliest vertebrates to have both innate and adaptive immunity, and acquired immunity is postulated to have evolved earliest in the gut of jawed fishes (Matsunaga, 1998; Matsunaga and Rahman, 1998; Cheroutre, 2004).
However, relatively few immunologists have focused their efforts on the study of mucosal immunity of fishes, and consequently, there is much less basic knowledge when compared to that known about the mammalian system. Also, necessarily, the experimental data generated from fish are in many cases more descriptive than mechanistic due to a paucity of immunological reagents available for quantitative studies (e.g., antibodies against cell surface antigens and signaling molecules, and knock-out, isogenic experimental animals) (Rombout et al., 1993; Lin et al., 1998; Huttenhuis et al., 2006).
For instance, although it is known that antigen is absorbed preferentially in the posterior intestine (Rombout et al. 1985; Georgopoulou and Vernier 1986; Otake et al., 1995), the precise sites where antigen is processed and presented by phagocytic cells, and where B and T lymphocytes interact, proliferate and differentiate remain unknown.
Relatively few cell-signaling molecules such as cytokines and chemokines have been identified. Lymphocytes and antibody-secreting plasma cells have been described in the intestinal epithelia and lamina propria (Rombout et al., 1993; Hebert et al., 2002), but the extent to which phagocytes and lymphocytes traffic between peripheral (mucosal) and central (pronephros and spleen) tissues is largely undetermined. Questions as basic as how antibodies produced at mucosal sites are translocated across intact epithelial cell layers also remain unanswered.
It is clear that compared to the substantial amount of experimental data that have contributed to the elucidation of the basic mechanisms of mucosal immunity in mammals, there is much less data available for fish. Most of the experimental work on basic immunity in fishes has focused on the systemic immune response, and what is known on mucosal immunity has been gleaned primarily from studies of the fish intestine, with less information available on the gills and skin.
As the elements of teleost mucosal immunity are presented in each section below, the mucosal immune response in mammals is briefly reviewed as necessary in order to point out the notable anatomical and functional differences (or similarities) that exist between the two groups. It should be emphasised, however, that contemporary fish have a mucosal adaptive immune system that is as effective in preventing infections as that of mammals. Comparative immunological studies are intended to shed light on evolutionary adaptations as well as provide insights into shared and unique mechanisms that exist among these different groups of animals.
Induction and Initiation of Mucosal Adaptive Immunity (Fig. 1.4)
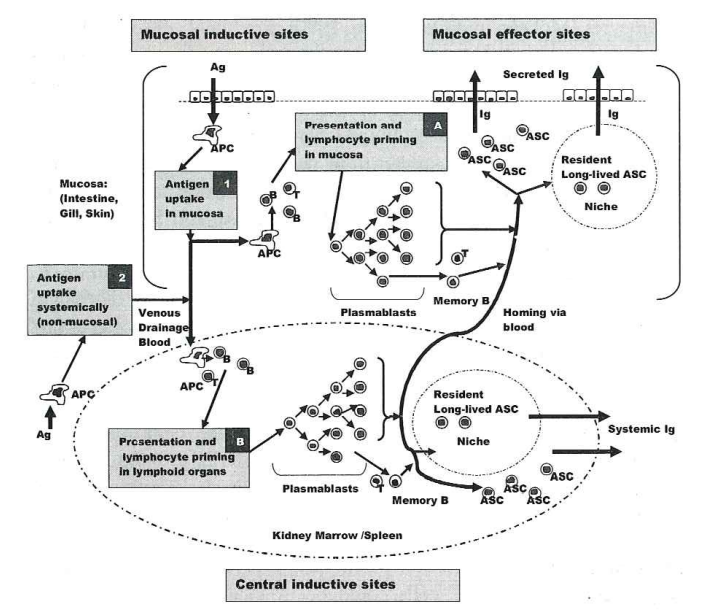
Fig. 1.4 Conceptualised elements of adaptive mucosal humoral immunity in teleosts.
In this model, which is derived from various studies in different fish species, both mucosal (1) and systemic (2) antigen (Ag) exposure are postulated to elicit a mucosal antibody (Ab) response. Mucosal exposure to antigen can elicit the production of systemic antibody as well. After entry through the mucosal epithelium or systemically (e.g., inoculation) antigen is phagocytosed by antigen-presenting cells (APC), processed, and presented in hypothetical mucosal inductive sites (A) and/or the central inductive sites of the pronephros kidney puip and spleen (B). Plasmablasts generated with T cell help in the kidney pulp and spleen traffic through the blood to peripheral mucosal sites. It is postulated that plasmablasts generated in mucosal inductive sites can traffic to central lymphoid organs as well. Following surface antigen exposure, mucosal antibody responses can be elicited without production of any systemic antibody. Memory B cells, long-lived antibody secreting cells (ASC), humoral memory and long-lived ASC niches are discussed in the text.
There is experimental evidence to suggest that the induction of mucosal immunity occurs by mechanisms similar to those that exist in higher vertebrates, namely, antigen processing and presentation by phagocytic cells, followed by priming of B cells and T cells, induction of B cell proliferation and differentiation with T cell help, and production of antibody by fully differentiated plasma cells (Miller et al., 1998).
The precise sites of antigen induction, however, and the degree to which the mucosal and systemic immune response interact, are still unknown. The sections below present current knowledge and hypotheses regarding the induction of mucosal immunity in the various mucosal tissues of teleosts.
Gastrointestinal Tract: The initiation of the mucosal immune response begins with uptake of antigen. The distal intestine of teleost fishes (referred to as the second intestinal segment) is the primary site of antigen uptake, and enterocytes in this region are postulated to function similarly to the specialised membranous epithelial cells (M cells) found in the gut of mammals (Davina et al., 1982; Egberts et al., 1985; Rombout and van den Berg, 1989; Rombout et al., 1989).
M cells, which are modified gut epithelial cells, serve as sites of antigen uptake (Egberts et al., 1985; McLean and Donaldson, 1990), and have apical membranes with microvilli that are shorter and broader than those on surrounding enterocytes (McLean and Donaldson, 1990), Epithelial cells with similar morphology have not been described in fishes, but the functional aspects of the posterior segment of the fish intestine suggest analogous roles for intestinal cells in this region, namely, the ability to absorb intact proteins and the close association of lymphoid cells (Rombout et al., 1985). Macrophages take up antigen from the posterior region of the gut, suggesting that this is a site of induction and initiation of the mucosal immune response (Rombout et al., 1985; Doggett and Harris, 1991).
Lymphocytes (referred to as intra-epithelial lymphocytes or IEL) are diffusely disseminated within the columnar epithelium (Rombout et al., 1993; McMillan and Secombes, 1997; Picchietti et al., 1997). These are primarily T cells, expressing the αβ T cell receptor (TCR), but a few antibody-secreting plasma cells are present as well (Scapigliati et al., 2000; Bernard et al., 2006).
Macrophages and lymphocytes also are distributed diffusely in the underlying lamina propria. Organised germinal centers functionally and morphologically comparable to the ileal and Peyer's patches and regional lymph nodes of mammals are not present.
Resident macrophages in the intestinal epithelium have been shown to take up antigen and display antigenic determinants on their outer membranes, suggesting an antigen-presenting function (Rombout and van den Berg, 1989). The differentiation and proliferation of resident or circulating antigen-specific B and T cells could occur locally following antigen presentation by resident macrophages, although this has not been shown experimentally.
The population of αβ T cells found in IEL populations were found to share functional and phenotypic similarity with aß T cells found in the peripheral circulation (Bernard et al., 2006), which allows the possibility that IEL circulate in the blood. It is also possible that following antigen uptake and processing (in the gut or elsewhere), antigen- presenting cells migrate to the central lymphoid organs of the pronephros (also referred to as the head kidney) and the spleen, where they subsequently present antigen to initiate the immune response (Rombout and Van den Berg, 1989).
This latter possibility would predict that differentiated T cells, plasmablasts or plasma cells that originate and develop in the central lymphoid organs traffic via blood to the peripheral epithelia. Again, there is no direct experimental evidence to resolve where the sites of induction occur. Studies indicate, however, that anal administration of particulate bacterial antigen elicits mucosal as well as serum antibody responses (Rombout et al., 1989).
Skin and Gills: The skin is the site where the immune system encounters most environmental pathogens (Kupper, 2000), and in mammals it has been postulated to serve as an immune organ (Puri et al., 2000). Mammalian skin has phagocytic dendritic cells (Langerhans cells) that extend pseudopodial processes between epithelial cells to reach close to the surface.
These cells survey the epidermal barrier for the presence of foreign antigen intrusion. Once an antigen is encountered, internalised and processed, Langerhans cells migrate to regional lymph nodes to continue their development, which involves the production of additional co-stimulatory molecules (involved in T-cell activation) and the cessation of antigen processing (Kupper, 2000).
The mature Langerhans cell no longer processes antigen to ensure that only the initial antigen encountered in the skin is displayed to initiate the immune response. Antigen is then presented to resident T cells, which when activated, home back to the skin in order to eliminate or prevent further antigen intrusion (Kupper, 2000). In mice, the epidermis also contains small numbers of specialised õy T cells, which are referred to as dendritic T cells. These cells have a restricted pattern of TCR usage and appear to play a unique role in cutaneous immune responses. Analogous cells are not found in humans (Bogen, 2004).
Fish have phagocytic cells and leucocytes that are associated with the epithelia of the skin and gills, either within the epithelium or immediately below it (Lobb, 1987; Iger and Wendelaar Bonga, 1994; Davidson et al., 1997; Lin et al., 1998; Moore et al., 1998), and these cells are postulated to be involved in the initiation of the mucosal immune response. Cells with the morphology of mammalian dendritic cells have not been described in fishes, but analogous antigen-presenting and processing cells are postulated to occur based on evidence such as the relatively high expression levels of MHC II B chain mRNA in gills of Atlantic salmon (Koppang et al., 1998).
However, the precise sites of induction of mucosal immunity are unknown. Studies in sea bass have shown that immersion vaccination elicits large numbers of antibody-secreting cells in the gills without a concomitant response in the gut or systemic organs (Dos Santos et al., 2001b). Similarly, it was shown in channel catfish that immersion vaccination in a soluble antigen elicited a mucosal antibody response without stimulating a serum antibody response (Lobb, 1987). These studies suggest that at some level, the development of the mucosal and systemic immune responses are partitioned, although it has been postulated that induction of an immune response at a particular mucosal site elicits stimulation in other remote mucosal tissues as well (Kawai et al., 1981, Rombout et al., 1989; Davidson et al., 1993).
Indeed, based on a number of studies in different fish species (St. Louis-Cormier et al., 1984; Rombout et al., 1989; Cain et al., 2000; Maki and Dickerson, 2003), there clearly appears to be cellular communication between mucosal and systemic induction sites following immunisation at either place. For example, antibody containing lymphocytes were increased in the skin of rainbow trout following the intracoelomic injection (i.c.) of sheep erythrocytes (St. Louis-Cormier et al., 1984). Similarly, i.c. injection of the major surface antigen of the parasite 1. multifiliis in channel catfish elicits both serum and cutaneous antibodies (Maki and Dickerson, 2003). Pathways of migration of antigen-presenting cells and lymphocytes within epithelia and among these tissues and the pronephros and spleen are postulated to occur as described above for the intestinal MALT. Research is necessary to elucidate more precisely the sites and kinetics of induction following exposure to antigen at different sites. The various possible sites of antigen presentation are shown diagrammatically in Fig. 9.4.
Effector Mechanisms of Mucosal Adaptive Immunity
The effectors of adaptive immunity are antigen-specific antibodies and cytotoxic T lymphocytes, both of which exist in teleosts.
While there is considerable experimental data regarding the molecular characterisation of antibodies and the kinetics of antibody expression, there is considerably less information available on antigen-specific cytotoxic T cell subsets (Nakanishi et al., 2002a). Most experimental work on T cells has focused on lymphocytes isolated from peripheral blood, head kidney (pronephros), or spleen.
Thus, the information presented below on the effector mechanisms of mucosal adaptive immunity is focused on mucosal antibodies, B cells and antibody-secreting plasma cells.
Mucosal Antibodies: The mucosal antibodies of mammals, which are predominantly dimeric molecules of the IgA isotype, are transported across epithelial layers to the mucosal surface by the polyclonal Ig receptor (pIgR) that binds the joining chain (J chain) of IgA and IgM molecules.
A part of the plg referred to as the secretory component is released together with the Ig into the mucosal secretions (Bogen, 2004). In teleosts, the predominant antibody found in both mucus and blood is an IgM tetramer with a molecular mass ranging from 600-900 kDa, with each monomeric subunit consisting of two light chains (each light chain polypeptide 25 kDa in size) and two heavy chains (each heavy chain polypeptide-70 kDa in size) (Wilson and Wart, 2002).
Although usually tetrameric in form under physiologic conditions, fish Ig has a degree of structural heterogeneity derived from non-uniform disulfide polymerisation of the monomeric or halfmeric (one light chain and one heavy chain) subunits (Kaattari et al., 1998; Bromage et al., 2004). This diversity is not related to isotypic differences (Bromage, 2005). Fish IgM is comparable to the pentameric mammalian IgM molecule with regard to heavy chain size, antigen affinity and avidity (Bromage, 2005). J Chains and plg receptors have not been reported in teleost fishes, except in one early study in a marine fish, Archosargus probatocephalus, in which a 95-kDa molecule was described covalently bound to the heavy chain of a dimeric Ig isolated from the cutaneous mucus (Lobb and Clem, 1981).
Recent studies in puffer fish (Takifugu rubripes) (Hamuro et al., 2007) and carp (Rombout et al., 2008), however, suggest the expression of plg receptors in skin and other mucosal tissues of teleosts, and a function in secretion of Ig. Although tetrameric IgM is the most common antibody produced in vivo among different fish species and the only isotype shown to be an effector of protective immunity, new isotypes recently have been discovered.
These include two transcribed u genes in salmon, & genes encoding IgD antibodies in salmon, channel catfish, cod and Japanese flounder, and o and t genes encoding IgZ and IgT in zebrafish and trout, respectively (Bromage, 2005). It has not yet been determined whether the functions of these isotypes occur in mucosal secretions. Twenty years ago, a fundamental question that remained unanswered in fish was whether or not mucosal antibodies are produced locally in the mucosa or remotely in the head kidney and spleen (Lobb, 1987).
Today, experimental evidence indicates that they are produced locally (Lobb and Clem, 1981a, b; Lobb, 1987; Rombout et al., 1993; Lin et al., 1996; Cain et al., 2000; Maki and Dickerson, 2003). For example, a localised cutaneous antibody response is generated against I. multifiliis, a protozoan parasite that infects the epithelial tissues of the skin and gills (Clark et al., 1992). Passive immunisation experiments with naïve channel catfish showed that mouse monoclonal antibodies (mAbs) against i-antigens confer protection against a lethal parasite challenge (Lin et al., 1996), but antibodies must be present at the site of infection.
Antibody availability and function depended on the molecular size of the antibody, as mouse IgG, but not IgM, antibodies protected. Similarly, serum antibodies from actively immune fish, which are tetrameric IgM-like molecules of approximately 750,000 daltons (Wilson and Warr, 1992), also failed to protect following passive transfer into naïve animals, despite the fact that such antibodies strongly immobilise the parasite in vitro (Lin et al., 1996). The ability to immobilise in vitro corresponds to protection in vivo (Clark et al., 1995).
These results indicate that antibodies must be present in the skin and presumably the gills where the parasite infects in order to afford protection. In further studies using the I. multifiliüs infection system, a two- to three-fold increase in IgM mRNA expression was demonstrated in skin at days 4 and 6 after I. multifilis invasion, signifying an upregulation of Ig transcription in response to infection (Sigh et al., 2004). These results suggest that antibodies are produced in the skin by resident antibody secreting cells (ASC). Additional experiments have shown directly that antibodies against 1. multifiliis are produced in the skin (Xu and Klesius, 2003). Skin explants removed from immune fish, and placed into sterile tissue culture media, produced I. multifiliis-specific antibodies, which persisted for four days, suggesting cells in the skin actively produced that specific antibody.
Cultures from skin explants of immune but not control-fish contained antibodies that immobilised 1. multifiliis and reacted with the predominant surface antigen on Western blots. In addition, similar experiments have shown that cutaneous antibodies against F. columnaré are detected in cultures of skin explants from infected channel catfish, suggesting that antibodies also are involved in protective immunity against this bacterial pathogen (Shoemaker et al., 2005). While experimental evidence indicates that mucosal antibodies are produced locally, the extent to which they differ in structure and function to serum antibodies remains unclear. Research has shown that cutaneous mucosal antibodies are physically and immunologically identical or share similar molecular epitopes to those isolated from blood (Lobb and Clem, 1981, 1982; St. Louis-Cormier et al., 1984; Itami et al., 1988; Rombout et al., 1993).
Studies in carp using mAbs against purified Ig from mucus or serum, however, have revealed antigenic differences between cutaneous mucosal antibodies and serum antibodies (Rombout et al., 1993). It has been suggested that alternate forms of Ig could be generated at mucosal surfaces that cannot be detected using current methods (Cain et al., 2000; Bromage, 2006).
B Cell Differentiation in Mucosal Tissues: In mammals, differentiation of B cells is initiated by antigen presentation in secondary lymphoid tissues such as lymph nodes, mucosa-associated lymphoid tissue (MALT), and spleen.
These lymphoid organs are organised to recruit naïve B and T lymphocytes from the blood and to promote their interaction with cognate antigen by activated antigen-presenting cells migrating to those sites from surrounding tissues. Once the lymphocytes have been activated and clonally expanded in centralised lymphoid organs, the resulting effector cells migrate to and localise in the infected or inflamed tissues.
For example, B cells responding to respiratory pathogens first are detected in local lymph nodes draining the respiratory tract, and later are found in the lung (Moyron-Quiroz et al., 2004). Mucosal surfaces are particularly vulnerable to infection, as these epithelial surfaces are thin and permeable barriers to the interior of the body, and the vast majority of infectious agents invade through these routes.
In mammals, mucosal-associated lymphoid tissue is organised to respond to pathogens invading through mucosal surfaces.
In fish, the skin, gills and intestine comprise the major surface areas of the animal exposed directly to the environment and are, consequently, the site of entry of many pathogens.
It is possible that lymphocytes and ASC directly underlying these surfaces serve as a primary site for antigen presentation to B cells, and consequently a site in which memory B cells differentiate and proliferate, facilitating rapid response to reinfection. Affinity maturation of antibodies is a cornerstone of the acquired immune response in mammals, and an increase in affinity of IgG antibodies by several orders of magnitude results from clonal selection of B cells (Gourley et al., 2004).
In teleosts, only IgM is produced and class switching does not occur. Whether affinity maturation and somatic hypermutation (SHM) of IgM occurs in fishes has been debated, but recent reports clearly demonstrate that modest increases in antibody affinity occur for trout IgM and shark IgNAR following immunisation with model antigens (Cain et al., 2002, Kaattari, 2002; Dooley, 2006). Sequence analysis of channel catfish heavy chain cDNAs has demonstrated SHM of both VH and JH encoded regions (Yang et al., 2006).
In mammals, activation-induced cytidine deaminase (AID) is an essential mediator of somatic hypermutation, class switch recombination, and gene conversion, all of which occur during the process of B cell differentiation and affinity maturation. AID is expressed exclusively in germinal centers and appears to be the only B-cell specific component required for these processes. It has been shown that AID is expressed in the skin of channel catfish suggesting that B cells may mature locally in the skin of this species (Saunders and Magor, 2004). Undifferentiated B cells responsive to LPS stimulation have been isolated directly from the skin of channel catfish (Zhao et al., 2008).
Immunological Memory and Mucosal Immunity (Fig. 1.4)
Activated B cells differentiate into populations of memory B cells and antibody secreting cells (ASC), which include plasmablasts, short-lived plasma cells and long-lived plasma cells.
In mammals, long-lived plasma cells reside in the bone marrow, where they produce the majority of circulating serum antibodies (Manz et al., 2002). Long-lived ASC may also occur in mucosal tissues (Etchart et al., 2006).
Long-lived plasma cells and memory B cells provide humoral immunological memory (Bernasconi et al., 2002; Gourley et al., 2004). Recent studies have provided evidence that subpopulations of antibody secreting lymphocytes, similar to those found in mammals, also occur in fishes (Bromage et al., 2004).
This work showed for the first time that long-lived ASCs reside in the head kidney of trout, and that these cells accumulate in this tissue and secrete antibody for as long as 35 weeks after immunisation. These cells are a source of serum antibodies. Such long-lived ASCs were not found in spleen or in the peripheral blood (PBL) population.
Short-lived (ie., weeks) plasma cells were found in both the spleen and head kidney. As stated earlier, tissues comparable to mammalian lymph nodes do not exist in fishes, and other than the spleen and pronephros, the anatomical sites where B cells encounter foreign antigen have not been well defined (Bromage et al., 2004).
It has recently been shown that B cells in fish also have potent phagocytic and micobicidal activities, not observed in mammalian B cells (Jun et al., 2006), suggesting that they play an even more central role in the initiation of immune responses than previously suspected.
These findings raise questions as to where primary adaptive immune responses occur following infection, and where memory B cells and long-lived plasma cells are generated and ultimately reside. It is possible, although not yet tested, that the skin, gills and intestinal epithelia with their associated lymphoid tissues are the primary sites of antigen presentation to B cells for epithelial pathogens.
They may not be the exclusive sites, however, as infection of the skin with I. multifiliis (as an example) leads to the production of antibodies in both the skin and serum, demonstrating that ASC localise to both skin and head kidney following infection (Maki and Dickerson, 2003). Nevertheless, it is possible that tissues directly underlying epithelial surfaces serve as sites for antigen presentation to B cells, and are consequently reservoirs for memory B and T cells, facilitating rapid response to re-infection, although as stated above, this remains to be tested. Whether or not long-term humoral immunity in mucosa is provided by long-lived plasma cells remains an open question in mammals (Etchart et al., 2006; Heipe and Radbruch, 2006).
ASC residing in nasal mucosa contribute to both serum antibody as well as secretory mucosal IgA. Their longevity suggests that survival niches for plasma cells exist in mucosal tissue and that these ASC constitute a second set of long-lived plasma cells (not residing in the bone marrow) that contribute to humoral immunity at mucosal surfaces. It is possible that an analogous situation exists in fishes as well. For example, channel catfish immunised against I. multifiliis remain immune to surface infection for more than a year, suggesting that protective cutaneous antibodies are continually produced by resident, long-lived ASC (Burkart et al., 1990; Zhao et al., 2008).
MUCOSAL IMMUNITY AND VACCINES
A recent survey of the fish farming community indicates that commercially available vaccines against 15 bacterial diseases are used worldwide in aquaculture (Hastein et al., 2005). The two main methods of vaccination are immersion and injection.
Oral vaccination is less effective compared to the other methods, although an experimental method has recently been developed using a plant expression system that may increase the efficacy of this route (Companjen et al., 2005).
Immersion vaccination with inactivated bacteria or subunit antigens is used against the following bacterial diseases (Hastein et al., 2005; Navot et al., 2005): classical vibriosis (Listonella anguillarum or Vibrio ordalii) in sea bass, salmonids, catfish, ayu, and turbot; furunculosis (Aeromonas salmonicida) in salmonids, spotted sea wolf and goldfish; yersiniosis (Yersinia ruckeri) in salmonids, cyprinids, eels, sole and sturgeon; pasteurellosis (Photobacterium damselae) in sea bass and seabream; warm-water vibriosis (Vibrio alginolyticus, V. parahaemolyticus, V. vulnificus) in barramundi, grouper, sea bass, seabream, and snapper; edwardsiellosis (Edwardsiella ictaluri) in channel catfish; flavobacteriosis (Flavobacterium columnare) in salmonids; flexibacteriosis (F maritimus) in salmonids and turbot; and streptococcosis (Streptococcus iniae) in rainbow trout, tilapia, turbot and yellowtail.
Viral vaccines licensed for aquaculture are all based on inactivated antigens in oil emulsions. Because the viruses or subunit components are non-replicating and non-infective, these vaccines are administered by injection (Biering et al., 2005). Antibodies are the primary response elicited following immunisation with these vaccines, which may not provide the most efficacious protection. Live attenuated viral vaccines comprise naturally occurring low-virulence isolates or virus that has been attenuated by other means.
The advantage of these types of vaccines is that they can infect by natural routes and replicate in the host. Thus, they can be administered either by immersion or orally. The primary disadvantage is the risk of reversion by mutation to virulent forms (Biering et al., 2005). Currently, however, no viral vaccines for fishes are administered by immersion (Navot et al., 2005). Vaccination by immersion has been used effectively to protect fishes against bacterial pathogens for many years, although the precise mechanisms of antigen uptake and protection remain unknown in many instances. It has been experimentally determined in some cases, however, that antigen passes through skin and gill epithelia directly or after hyperosmotic and/or ultrasound treatment to reach the blood and lymphoid tissues (Alexander et al., 1982; Ototake, 1996; Ototake et al., 1996; Moore et al., 1998; Navot et al., 2004).
Ultrasound irradiation causes microscopic injuries to the skin (Navot et al., 2004, 2005), and it has been suggested that this treatment is comparable to intradermal immunisation, which in mammals is one of the most effective means of vaccination (Navot et al., 2005). Uptake also is enhanced following mild, controlled puncture or abrasion of the skin (Nakanishi et al., 2002b). Immersion vaccines against pathogens that gain entry through the gill or skin epithelia have been effective when antibodies against surface antigens are elicited that block pathogen entry and colonisation. For example, immersion vaccines against Photobacterium damselae subspecies piscicida (formally Pasteurella piscicida) comprised of the over-expressed 97- kDa and 52-kDa bacterial proteins are effective with relative percentage of survival (RPS) rates of 50% when compared to controls (Barnes et al., 2005).
Following immersion immunisation of sea bass, the gills were shown to be the primary sites for ASC, indicating that protective antibodies are produced (and perhaps stimulated) locally (Dos Santos et al., 2001b; Barnes et al., 2005). A non-commercial, experimental subunit vaccine comprised of the major surface antigen of I. multifiliüs elicits a cutaneous antibody response and protective immunity against challenge (Wang and Dickerson, 2002; Wang et al., 2002).
Adjuvants and Delivery Methods That Enhance Mucosal Immunity: Adjuvants are compounds that aid immunity through accelerated, prolonged or enhanced responses to vaccine antigens. Although many different adjuvants have been tested in fish (mainly through trial and error), water-in-oil immersions in either mineral or non-mineral oils have proved to be the most successful in commercial aquaculture (Schijns and Tangeras, 2005). There is little information available in the literature on adjuvants and mucosal immunity, however. Approaches used in the human vaccinology field include the use of toll-like receptor agonists (e.g., CpG motifs and gylcans), as well as immunostimulants (e.g., cytokines and co-stimulatory molecules such as interleukin) (Toka et al., 2004). ADP- ribosylating toxins have been used as effective mucosal adjuvants in higher vertebrates, but have not yet been tested or established as mucosal adjuvants in fishes. A number of treatments (hypo- and hyper-osmotic baths, scarification of skin surfaces, ultrasound irradiation and combinations of hyperosmotic dips and ultrasound irradiation) have been used in combination with antigen immersion to enhance mucosal immune responses. These have been referenced in the preceding section.
'The biology of teleost mucosal immunit' 카테고리의 다른 글
| The biology of teleost mucosal immunity- INTRODUCTION (0) | 2025.01.05 |
|---|