PART ONE: THE DIFFERENT CELL TYPES IN FORMALIN-FIXED, PARAFFIN-EMBEDDED TISSUES
EPITHELIAL CELLS
Epithelial cells are the most abundant in the body and have many important functions. For example, they cover our skin and line the gastrointestinal tract from top to bottom. They are also the progenitor cell for what is by far the most common type of cancer in humans: the carcinomas. The different types of epithelial cells are easy to recognize under the microscope.
Squamous Cells
A major function of squamous cells is protection. They protect the skin from evaporation and from outside forces that could puncture the skin and, thus, cause bleeding. They produce, in abundance, a protein that is highly characteristic of this cell type: cytokeratin. Figures 6-1 and 6-2 show the variability that squamous cells can show in formalin-fixed, paraffin-embedded tissues. Let’s first focus on what these different images have in common, which are the defining characteristics of squamous cells in general:
1. They have a thick, stratified layer of cells.
2. The mature squamous cells toward the surface are large and flattened.
3. Squamous cells are linked by bridge-like tight junctions.
Figure 6-1, panel A, shows the stratification of these epithelial cells very clearly. As you progress to higher magnification, the large flattened cells at the surface and the tight junctions (arrows, panel D) are clearly evident. Clearly, having such a thick layer of cells in which each cell is holding tightly to its neighboring cells will result in a watertight barrier that can withstand the bumps and bruises of everyday life.

Figure 6-1 Epithelial cells—the stratified squamous cells: part A. Panel A shows the stratification of these epithelial cells very clearly. The cells are dark blue and are layered like tiles in about 20 separate rows over the underlying dermis. Note that the dermis is mostly dark pink, owing to the collagen that gives the skin its strength. As you progress to higher magnification, the large flattened cells at the surface and the tight junctions that hold the squamous cells together (arrows, panel D) are evident. Clearly, having such a thick layer of cells in which each cell is holding tightly to its neighboring cells will result in a watertight barrier that can withstand the bumps and bruises of everyday life.
Figure 6-2 also shows squamous epithelia; each of the three features noted previously are evident. However, note that in panel A the squamous cells have large, clear zones. In comparison, panels B–D show, at successive magnifications, that the squamous epithelial cells are lined by a dark pink layer. Before we get into why panel A looks so different from panels B–D, let’s remember some basics about hematoxylin and eosin stains that we discussed in previous chapters:
Hematoxylin stains nucleic acids blue. Thus, the blue stain typically corresponds to the nucleus. Eosin stains proteins pink. Thus, the pink stain typically corresponds to the cytoplasm.

Figure 6-2 Epithelial cells—the stratified squamous cells: Part B. This figure also shows squamous epithelia. However, note that in panel A the squamous cells have large, clear zones. In comparison, panels B–D show, at successive magnifications, that the squamous epithelial cells are lined by a dark pink layer. Panel A represents an example of infection by human papillomavirus (HPV). HPV primarily infects squamous cells and causes the cytoplasm to fill with water. This pushes the organelles and cellular proteins to the side. Since the water has no color, the end result is this clear zone called a koilocyte. In comparison, panels B, C, and D show the most typical feature of squamous cells besides their multiple stacked layer pattern of growth. Squamous cells make keratin, and it gives them their strong pink color. If the tissue is under any kind of pressure stress (as occurs routinely on the hands and feet), then the squamous cells will mature into a thick, protective outer coat of keratin. That is why this outer layer is dark pink (panel B, black arrow). The yellow arrow in panel B also marks a thick protein layer, but this is the dermal collagen. Finally, note that in panels C and D the squamous cells at the surface of the skin (left part of image) have small inconspicuous nuclei as compared to the deeper layer of squamous cells. This is called parakeratosis and simply refers to the persistence of the nuclei in the squamous cell as it matures into a small “ball of keratin” on the skin’s surface.
Let’s examine again Figure 6-2, panel B. Note that it has two dark pink zones (small arrow, surface, and larger arrow, at the base of the epithelia). Also note that the squamous cells between the two arrows have blue nuclei and a light blue cytoplasm. So, from what we just noted, we can make the following statements:
1. The material highlighted by the arrows is a densely packed protein.
2. Toward the base and middle, the squamous cells show some nucleic acids in their cytoplasm, but toward the surface their cytoplasm contains mostly protein.
What are the densely packed proteins indicated by the arrows? The concentrated protein marked by the small arrow at the surface is keratin. Keratin is an excellent water proof agent.
There are very common diseases of the skin in which the keratin breaks down, and thus, the person gets small blisters, or “bumps,” that tend to ooze fluids due to the loss of this protein barrier. Two common diseases that fit this category are eczema and contact dermatitis (e.g., poison ivy). The other protein (large arrow) is called collagen. It is the main protein of the stroma, which we discuss later.
Finally, it makes sense that the squamous cells in Figure 6-2, panels B–D, will show RNA (nucleic acids) in their cytoplasm toward the base of the skin, because these cells make the RNAs that are needed for keratin synthesis.
Of course, at the surface, the cells don’t need RNAs but need the protein for their protection, and thus, the cells at the surface actively make the protein. Getting back to Figure 6-2, the tissue in panel A is from the cervix, whereas in panels B–D it is from the hands. There is no “need” to protect the cervix with abundant keratin, whereas the skin of the hand is constantly active and, thus, needs much more protection against the elements.
Mucosal squamous epithelia are, thus, sometimes referred to as “nonkeratinizing” epithelia, whereas skin epithelia are sometimes called “keratinizing epithelia.” I do not like these terms because mucosal squamous epithelia do make keratin, and we can use that to our advantage with immunohistochemistry. They simply make much less keratin than the squamous epithelia of skin.
It is amazing how specialized squamous cells can be! Of course, they form the nails of our fingers and toes, as well as our hair. In the animal kingdom, these cells are responsible for hooves, antlers, feathers, scales, and so on! As with most proteins, when they are abundant and concentrated enough, they can form a very strong structure indeed!
Glandular Epithelia
Whereas squamous cells are analogous to the tile on our roof or the armor of a tank, in the sense that one of its main roles is protection, glandular epithelial cells are more involved with direct physiologic functions. This is not to say that squamous epithelia are inert in this regard. But, in general, glandular epithelial cells do not play any protective role but rather have a functional role.
The one exception would be that glandular cells can make a substance called mucin that can play an important lubricating and protective role in certain organs, such as the stomach. Glandular cells line our gastrointestinal tract. They also make up the major digestive organs such as the pancreas and gallbladder. They can have an endocrine function evident in the thyroid, which is a modified gland based in glandular epithelia. Here are the salient features of glandular epithelia and, thus, how we can recognize them in the hematoxylin and eosin:
1. Single layer of cells
2. Large columnar shaped cells
Figure 6-3 shows three tissues where glandular epithelia are present: the colon, the cervix, and the endometrium. Note that at low magnification (panels A and C) the glands consist basically of round to oval tubes. This gives the pattern of many tubes arranged in a well-organized, uniform pattern.
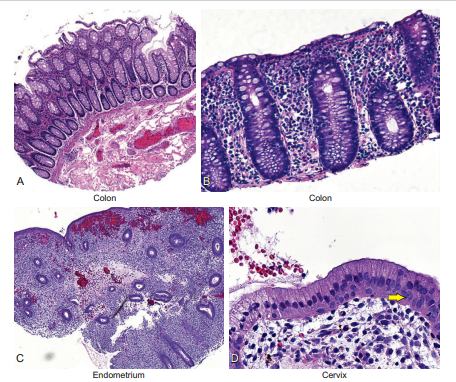
Figure 6-3 Epithelial cells—the glandular cells: Part A. This figure shows the glandular epithelia found in the colon (panels A and B), endometrium (panel C), and cervix (panel D). Note that at low magnification (panels A and C) the glands consist basically of round to oval tubes. This gives the pattern of many tubes arranged in a well-organized, uniform pattern. Well-organized glands are always benign. Note that in panel D the cervix shows the single-cell columnar lining typical of glandular epithelium. However, as noted by the arrow, smaller cells are present at the base of the gland. These are the so-called reserve or stem cells. These are the cells that can make new glandular cells when the older ones undergo the normal process of cellular turnover. They are also capable of making squamous cells in a process called squamous metaplasia. Finally, note that the glandular cells in panels A, B, and D are tall and show a gray color, whereas the glandular cells in panel C are smaller and don’t have this gray material. The gray material is the mucopolysaccharides that these cells make as a necessary lubricant in the colon and cervix. The endometrial cells at this stage (called the proliferative stage) do not need to make any secretions; this happens later in the endometrial cycle.
Pathologists love to be descriptive about such patterns. One such term they use for this simple uniform pattern of many glands arranged next to each other is a “wallflower” pattern of growth. Note in panel D that the cervix shows the single-cell columnar lining typical of glandular epithelium. However, as noted by the arrow, smaller cells are present at the base of the gland. These are the so-called reserve or stem cells. These are the cells that can make new glandular cells when the older ones undergo the normal process of cellular turnover. Glandular epithelia, as noted previously, can take on major functional roles.
This is clearly evident in the pancreas and thyroid. The pancreas must make most of the digestive enzymes needed to break down the food we eat. One of the many serious problems children with cystic fibrosis face is that their pancreas is destroyed by the disease. Therefore, they cannot properly break down the food they eat for energy and nutrients and, thus, must take digestive enzyme supplements. In the glandular lined organs where the cells have a major secretory function, it is common for the cells to acquire a lot of cytoplasm and form small clusters of cells. These clusters, called acini, are depicted in Figure 6-4 for the breast and pancreas.
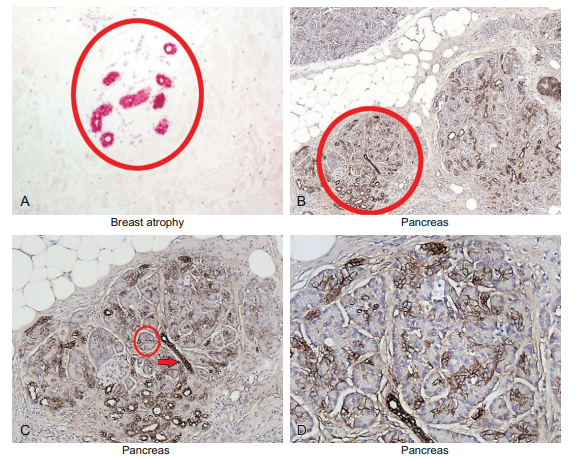
Figure 6-4 Epithelial cells—the glandular cells: Part B. Glandular epithelia are responsible for many diverse functions. This includes the production of many secreted products such as milk (breast, panel A), and the digestive enzymes needed to process the energy from food (the pancreas, panels B–D). Breast and pancreatic epithelia are each patterned as small outpouchings of glandular epithelia (called acini) surrounding the small ducts that drain these acini. In the breast these acini atrophy over time. This will produce an atrophic pattern where the small ducts persist but the acini are gone (panel A, highlighted by the keratin immunohistochemical stain). In comparison, look at the pancreas in which atrophy does not occur over time. The glands are arranged in circular lobules (panel B). The lobules, in turn, are composed of ducts (highlighted by the cytokeratin AE1/3 stain as in panel A). The glandular epithelia around the ducts (called the acini; panel C, circle with a red arrow at the duct) do not stain well with AE1/3. The acini cells are responsible for the massive secretions that the pancreas must make daily. As an aside, the duct is responsible for most pancreatic tumors in particular and adenocarcinomas in general. This is why adenocarcinomas are typically positive for AE 1/3.
We can think of this as single layer of tall cells where the main duct of the gland made many small buds lined by these cells to maximize the amount of secretory product in a relatively small area. Glandular epithelia are the progenitor of all the adenocarcinomas. These are among the most common and lethal tumors and include, of course, most colon, breast, prostate, ovarian, uterine, pancreas, and gastric cancers, and many lung cancers. Since this chapter is being written primarily for the nonpathologist, I am oversimplifying some points. Some benign glands can show stratification. The best example is the endometrium, and it reflects the high mitotic activity the endometrium shows during the proliferative phase. But, in general, the points listed here are accurate and will help you get a base of knowledge to build upon.
Transitional Epithelia
It is easy to remember the features of the last type of epithelia, transitional epithelia. The reason is that it represents a transition from the single-layered, tall glandular cells to the multilayered, flattened squamous cells. Transitional epithelia are only found lining the urinary bladder where these cells routinely face high intraluminal pressures. Thus, a single layer of epithelia would not be sufficient to protect the underlying blood vessels from trauma. Rather, the defining characteristics of transitional epithelia (also known as urothelial epithelia) are as follows:
1. There is a thin, stratified lining of cells
2. The cells at the surface have small caps
The histologic features of transitional epithelia are shown in Figure 6-5.
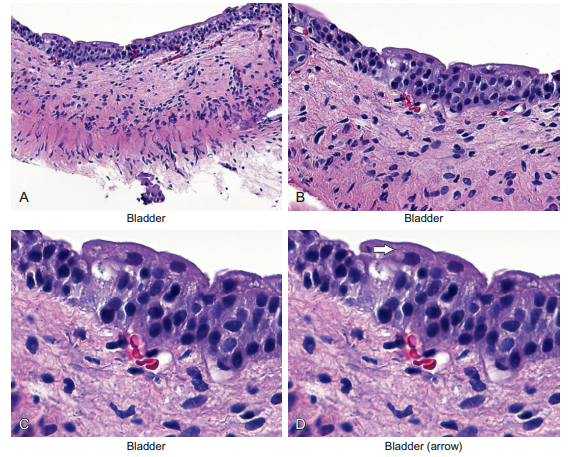
Figure 6-5 Epithelial cells—the transitional cell. The bladder is lined by epithelial cells that are somewhere in between the thick layers of squamous cells and the single layer of tall cells of glandular epithelia. Logically, these cells are called transitional cells because they represent a transition between these two disparate epithelial cell types. Note that the bladder tissue is lined by about five rows of transitional cells stacked on each other. No keratin is present or “needed” since the surface of the bladder is not under the kind of pressure that the skin is under. In panel D note the large cap-like cells on the top that form an umbrella-like structure over the other urothelial epithelial cells. This feature of differentiation is any easy way to be sure that the epithelia are benign.
Note that the arrow in panel D highlights the large cap-like cells on the top that form an umbrella-like structure over the other urothelial epithelial cells. Thus, these cells represent the most differentiated form of urothelial cells. Recognizing these umbrella cells is very useful in surgical biopsies. The reason is that malignant cells rarely form the most differentiated form of the epithelia from which they were derived. So the presence of these cap or umbrella cells in a bladder biopsy will reassure the pathologist that the lesion is probably benign. A better example of this rule is cilia. The terminal hair-like cilia on a cell is typical of glandular epithelia that need to move something along a corresponding “channel.” Good examples of this include the fallopian tube and the lung, which must move the fertilized egg and mucus, respectively. Malignant cells simply do not like to differentiate and, in my experience, there has been no proven example of a malignant tumor that contained cilia. This can be very helpful when interpreting atypical cells in a lung washing.
STROMAL CELLS
It is always easy to find stromal cells after locating the epithelial cells. The stromal cells make up the tissue that is underneath the epithelia. In the skin, the stroma is commonly called the dermis. In the gastrointestinal tract, the stroma is often called the lamina propria (right under the epithelia) or submucosa (deep below the epithelia).
To better understand what cells we will find in the stroma, let’s think of its function:
1. To support the overlying epithelia
2. To deliver blood and nutrients to the tissues
3. To help remove the waste products from the tissues
4. To help in the immune protection of the tissue
The support function of the stroma is done mostly by one cell: the fibroblast. Its job is to make the main protein of the stroma, collagen, which we discussed regarding Figure 6-1.
The delivery and removal of nutrients/waste products is done by small blood vessels that are lined by one simple cell type: the endothelial cell. The small vessels are called the capillaries and the lymphatic channels.
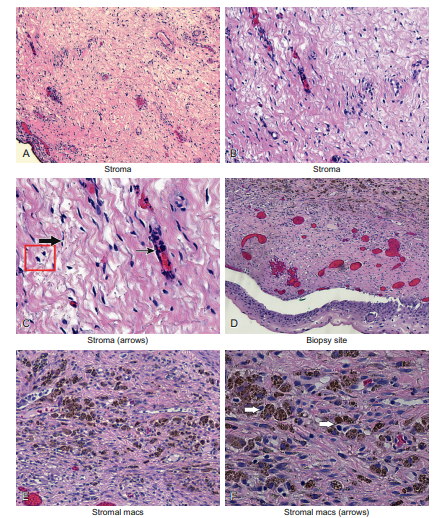
The cells in the stroma that are the “protectors” against foreign invaders are called macrophages. Therefore, recognizing the specific cell type in the stroma is not difficult if you remember that, for the most part, all you need to be able to do is to discriminate the fibroblast from the endothelial cell from the macrophage. These points are illustrated next and in Figure 6-6.
Fibroblasts
1.Large, isolated cells with elongate prominent nuclei
2. Associated with extracellular dark pink protein (collagen)
Endothelial cells
1. Large, very flat cells with inconspicuous nuclei
2. Line microvessels (capillaries, which contain red blood cells, or lymphatics, which contain clear fluid)
Macrophages
1. Large cells that tend to form small clusters with round to oval nuclei
2. May contain foreign pigment and/or hemosiderin pigment
In Figure 6-7, panel C, the fibroblast is depicted with a large arrow, the endothelial cell with a small arrow, and a few macrophages are shown inside a small box. Panels D–F are successive magnifications of a cervical biopsy from a woman who recently had a separate cervical biopsy. Therefore, as expected, there was a lot of bleeding. This stimulated the macrophages to proliferate. They are easily seen in panel F, where their abundant large brown pigment is evident in the cytoplasm (arrows). This is the hemosiderin pigment, and is the byproduct of the bleeding that occurred in this area after the initial biopsy.
INFLAMMATORY CELLS
It would be unusual to see inflammatory cells if you biopsied normal tissues. Of course, by definition, surgeons do not routinely biopsy normal-appearing tissues. Therefore, in surgical biopsies you usually encounter inflammatory cells. There are four major types of inflammatory cells that we need to recognize when interpreting immunohistochemistry or in situ hybridization results:
1. Lymphocytes 2. Plasma cells 3. Eosinophils 4. Neutrophils
Lymphocytes
Lymphocytes are the most common of the cell types we will come across when interpreting immunohistochemistry or in situ hybridization data. Panels A–D in Figure 6-8 show successively increasing magnifications of a cervical biopsy. The majority of cervical biopsies for an atypical Pap smear show lymphocytic infiltration. Here are the features that allow us to identify the lymphocytes under the microscope:
1. Aggregates of small, round cells with dark nuclei and scant cytoplasm
2. Typically large numbers owing to the fact that some antigenic stimulus is “calling” them to this site
There are many subtypes of lymphocytes. In general, they fall into the category of T-cell, B-cell, natural killer (NK) cell, and memory cell. In each group are many other subcategories. In most cases in which you perform immunohistochemistry or in situ hybridization, the only knowledge you need is the general category. As we discuss in a moment, each category has its own specific antigenic marker that allows us to characterize it accurately.
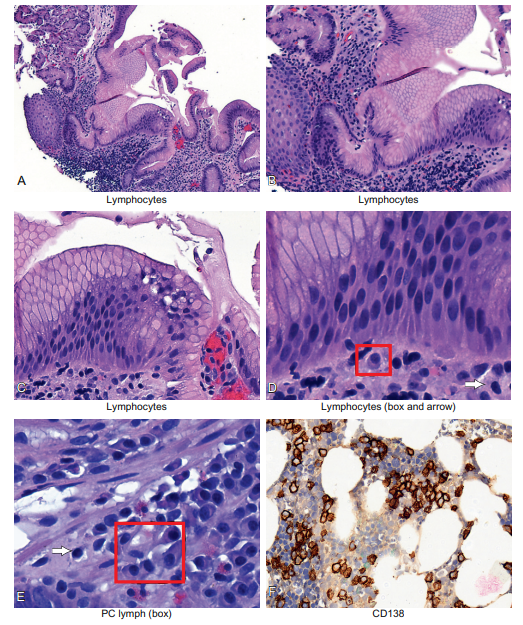
Figure 6-8 The inflammatory cells: the lymphocyte and the plasma cell. This figure is from an esophagus with chronic esophagitis. Note the large collection of small blue cells in the stroma under the glandular epithelia in panels A, B, and C. Also note in panel C that there is a collection of stratified squamous cells in the middle of the epithelia. This replacement of the glandular cells by the squamous cells in the cervix is normal and is called squamous metaplasia. Squamous metaplasia is the initiation point of HPV in the cervix. Panels D and E show at higher magnification the plasma cells (box) and the lymphocytes (arrow). The plasma cells show eccentric nuclei, a clear zone around the nucleus, and a bluish-pink cytoplasm. Plasma cells, which are ultimately responsible for making all the antibodies we use in immunohistochemistry, are small protein factories; the bluish-pink cytoplasm reflects the high mRNA and protein content of these cells directed to producing the target-specific antibody. Panel F shows an antigen called CD 138 that is present in most plasma cells. This is from a person who had a malignant tumor of the plasma cells called multiple myeloma. Not surprisingly, many symptoms these patients get relate to the high number of antibodies and proteins that the tumor cells synthesize.
Plasma Cells
As indicated previously, when you are analyzing a biopsy and see there is inflammation, in most cases the lymphocytes will dominate. They are easily recognized as aggregates of small, round cells with dark blue nuclei and very little cytoplasm. These cells represent the body’s attempt to deal with some relatively long-term exposure to a foreign antigen. The inflammation takes many days to start up and usually longer to leave. Hence, it is often referred to as chronic inflammation.
Acute inflammation, which we discuss shortly, is much less common and usually reflects either acute trauma or the rapid response to the presence of a bacterial infection. Once the chronic inflammation is established, the body will try to rid itself of the foreign antigen. One way it will do this is by producing antibodies. Some lymphocytes, called B-cells, will gain the ability to synthesize specific antibodies against the foreign antigen. These cells become metabolic machines, geared to the production of large amounts of antibodies. These antibodies are stored in the cytoplasm. Thus, these cells, called plasma cells, can be readily distinguished from lymphocytes by their ample cytoplasm. Here are the features that allow us to identify the plasma cells under the microscope:
1. Aggregates of larger, round cells have open nuclei and ample cytoplasm.
2. The chromatin of the nuclei often forms small clusters around the periphery of the nucleus, hence the term “clock-wheel chromatin.”
Examples of plasma cells are shown in Figure 6-8, where they are enclosed in small boxes. In comparison, the smaller lymphocytes are marked by the arrow.
Eosinophils
Eosinophils are another inflammatory cell type of chronic inflammation. Their presence usually denotes an allergic type response. Hence, they are very commonly seen in diseases such as asthma. Their presence can be diagnostic of specific diseases. For example, there is a disease called eosinophilic esophagitis in which the presence of at least 15 eosinophils per high power field is sufficient to render this diagnosis. Here are the features that allow us to identify the eosinophils under the microscope:
1. Aggregates of intermediate-sized, round cells have open nuclei and ample cytoplasm.
2. The nuclei have two lobes, and the cytoplasm has small pink granules.
Figure 6-9 presents images from a case of eosinophilic esophagitis. Panels A through D show the histology at successively high magnifications. Note in panel D that the esophagus is lined by stratified squamous epithelia. Also note that interspersed among the large squamous cells are many smaller inflammatory cells with bilobed nuclei and ample cytoplasm that contains pink granules. The latter gives the eosinophils their bright red color and, of course, their name because they stain strongly with eosin.
Neutrophils
Whereas lymphocytes, plasma cells, macrophages, and eosinophils represent the chronic reaction of the body to a foreign antigen, neutrophils represent an acute reaction to some antigen. Common examples of acute inflammation include exposure to bacterial antigens, and the acute death of cells, such as after radiotherapy, chemotherapy, or ischemia (such as a heart attack). Tissue ulceration is another example where there is acute cell death and tissue damage that results in the infiltration of the tissue by neutrophils.
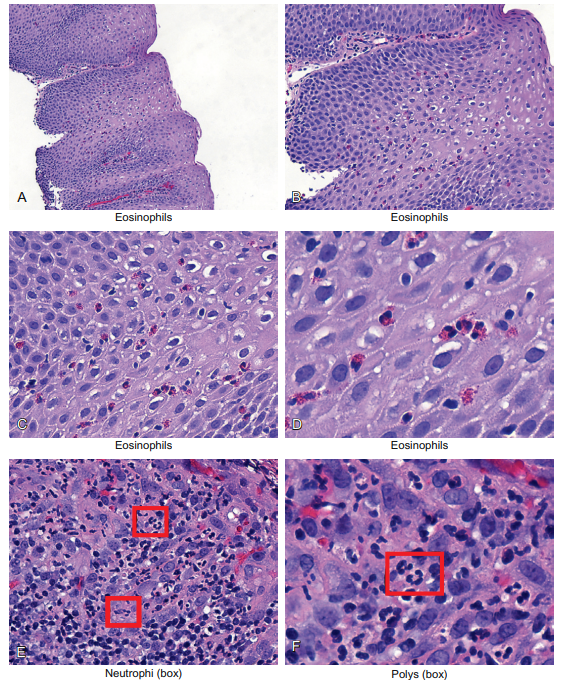
Figure 6-9 The inflammatory cells: the eosinophils and neutrophils. Panels A through D show the histology at successively high magnifications; the tissue is the esophagus from a young person who had difficulty swallowing with heartburn that he indicated was related to specific foods. Note that the esophagus is lined by stratified squamous epithelia. Also note that interspersed among the large squamous cells are many smaller inflammatory cells with bilobed nuclei and ample cytoplasm that contains pink granules. The latter gives the eosinophils their bright red color and, of course, their name because they stain strongly with eosin. Panels E and F show the stomach of another person with acute gastritis associated with marked reflux. Note that many small cells are infiltrating the epithelia. They have multilobed nuclei (box, panel F) that give these cells their name; polymorphonuclear leukocytes (polys for short). They are also called neutrophils. Their presence is classic of acute tissue damage and bacterial infection.
Figure 6-9 shows some examples of neutrophilic infiltration; the lesion was a stomach ulcer. Here are the features that allow us to identify the neutrophils under the microscope:
1. Aggregates of intermediate-sized, round cells have open nuclei and ample cytoplasm.
2. The nuclei have multiple lobes, and the cytoplasm has small weakly staining granules.
The neutrophils in Figure 6-9 are marked by the small boxes. Note the multilobed nuclei that are the pathogenomic feature of these cells. Sometimes these nuclei look like the hat worn by Mickey Mouse, hence the term “Mickey Mouse nuclei”! As I said, there seems to be no limit to the descriptive ability of pathologists!