TERMS COMMONLY USED TO DESCRIBE FORMALIN-FIXED, PARAFFIN-EMBEDDED TISSUES
There are terms that you will need to become familiar with when interpreting either your own or other people’s data using either in situ hybridization or immunohistochemistry. These terms are discussed next.
ATROPHY
Atrophy refers to the thinning of a specific type of tissue. It usually refers to epithelia. Since the epithelia are thinned, by definition, there are far fewer cells per unit area of the biopsy. The most common cause of atrophy is simply aging. Another relatively common cause is the re-epithelialization of a tissue after ulceration or after a biopsy. Another less common cause is chemotherapy effect.
Figure 6-10 shows two different examples of atrophy.
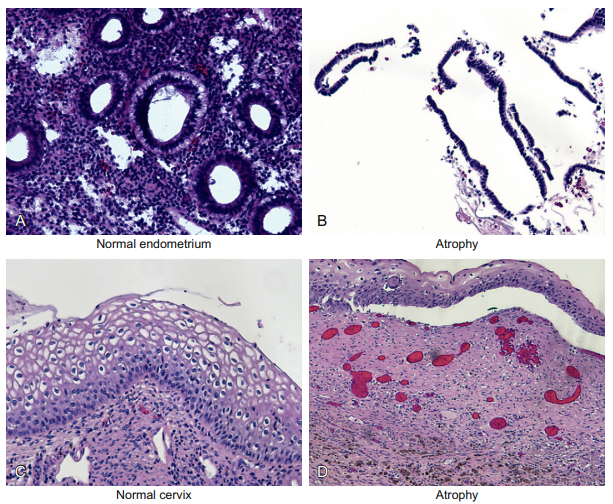
Figure 6-10 Epithelial cells and atrophic changes. This figure shows two different examples of atrophy. Panel A is a normal endometrial biopsy from a 25-year-old woman. Note the abundant glands and stroma. Panel B is the endometrial biopsy for an 81-year-old woman. Note that the stroma is so atrophic that it is barely visible and that the epithelia consist of far fewer cells than is evident in panel A. This is the end result of the lack of estrogen stimulation on the endometrium, and reminds us that epithelia are not static but always undergoing cell turnover. Panel C shows a squamous epithelial lining of a cervix in a 32-year-old woman. Compare this to panel D, which is the cervical biopsy of a 30-year-old woman who had a large cervical biopsy taken a few weeks before. The ulceration caused by the biopsy is now re-epithelializing, and the squamous lining is still much thinner than normal. Thus, this is a different cause of atrophy—the loss of the surface epithelia from, in this case, a biopsy and the subsequent re-epithelization of the lining.
Panel A is a normal endometrial biopsy from a 25-yearold woman. Note the abundant glands and stroma. Panel B is the endometrial biopsy for an 81-year-old woman. Note that the stroma is so atrophic that it is barely visible and that the epithelia consist of far fewer cells than is evident in panel A; therefore, the glands are also atrophic. Panel C shows a normal squamous epithelial lining of a cervix in a 32-year-old woman. Compare this to panel D, which is the cervical biopsy of a 30-year-old woman who had a large cervical biopsy taken a few weeks before. The ulceration caused by the biopsy is now re-epithelializing, and the squamous lining is still much thinner than normal. Therefore, it is still atrophic. An important corollary for atrophic cells and immunohistochemistry plus in situ hybridization is that atrophic cells tend to be metabolically inactive. Therefore, they often do not show the robust protein/DNA/RNA synthesis required for a strong signal with any of our in situbased molecular pathology methods.
ULCERATION/EROSION
Whereas atrophy is the thinning of tissue, and often refers to the epithelial layer, ulceration is the loss of the epithelial lining. The lining is usually lost because the epithelial cells have been actively destroyed.
If the epithelial lining is much thinned but not completely lost, it is called an erosion. Of course, ulcerations expose the underlying blood vessels in the stroma. Thus, most ulcers readily bleed.
The bleeding will be associated with hemosiderin deposition in the tissue. Also, the patient will often show a decreased red blood cell count, referred to as a decreased hematocrit. Panels A and B of Figure 6-11 show low and high magnifications of the ulceration found in a person with Barrett’s esophagus.
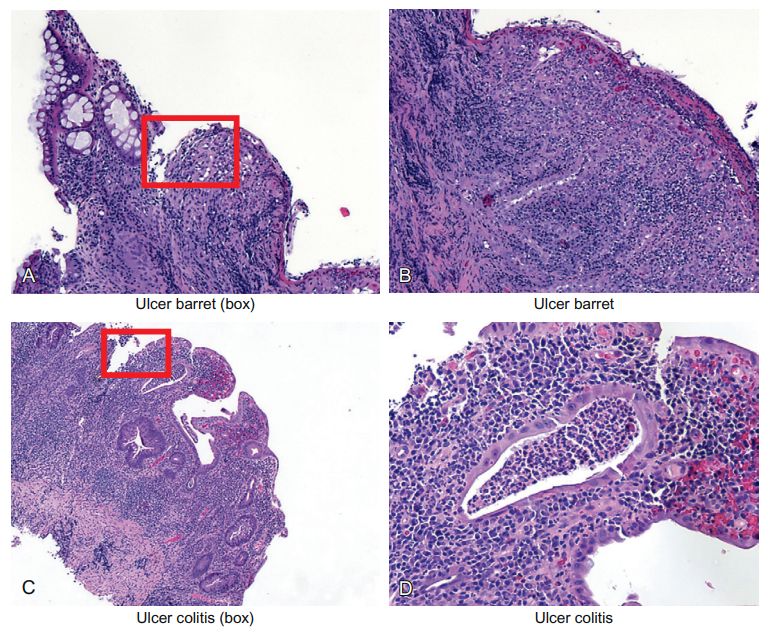
Figure 6-11 Epithelial cells and ulceration. Ulceration is defined as the complete loss of the epithelial lining of any tissue; a partial loss is called an erosion. In either case, severe, typically acute, inflammation eventually destroys the epithelial lining but not the stroma. Panels A and B show low and high magnifications of the ulceration found in a person with Barrett’s esophagus. This disease is marked by the replacement of the normal thick squamous lining with a much thinner glandular lining. Since the glandular lining often has mucusproducing cells that look like the intestine, it is called intestinal metaplasia. The area where the epithelial lining is lost is marked by a box. Similarly, panels C and D show the marked acute and chronic inflammation with partial loss of the epithelial lining of the colon marked by the disease called ulcerative colitis. The area of ulceration is marked also by a box. Also note the collection of neutrophils in the tubular gland (center of image, panel D). This is called a crypt abscess; an abscess is simply a collection of neutrophils. Also note that the inflammation is spilling over into the stroma. This will lead to the destruction of the small blood vessels. Since the function of the colon epithelia is to absorb water, patients with this horrible disease get bloody diarrhea and cramps.
This disease is marked by the replacement of the normal thick squamous lining with a much thinner glandular lining. Hence, these people tend to get ulcers. These tissues also are both acutely and chronically inflamed. As we discuss later, the presence of chronic inflammation per se predisposes the site to cancer. Indeed, people with this disease have a much increased risk of esophageal cancer.
The area where the epithelial lining is lost is marked by a box. Similarly, panels C and D show the marked acute and chronic inflammation with partial loss of the epithelial lining of the colon marked by the disease called ulcerative colitis. The area of ulceration is marked also by a box. Unfortunately, besides the horrible symptoms, people with this disease also have an elevated risk of colon cancer.
Since the epithelial cells with ulcers not due to old age are actively trying to repair the damaged lining, they tend to be very metabolically active. This allows us recognize them easily.
Specifically, atrophic cells tend to have scant cytoplasm and inactive-appearing (pale, uniform) nuclei. In comparison, cells trying to repair an ulcer tend to have large, open nuclei because they are turning on many RNA and protein synthesis pathways. They also have large structures in the nucleus called nucleoli. These structures appear as large dots in the nucleus.
The nucleoli is the center of ribosomal RNA synthesis, which, in turn, is important for the many messenger RNAs (mRNAs) the cell will need to make as it tries to repair the area of injury. Nucleoli are formed in the nucleus around part of the chromosome called NORs (short for nucleolar organizing regions). NORs are composed of many consecutive repeats of rRNAs and are found on several different chromosomes.
These are quiescent in the mature epithelial cell (or stromal cell), but can be “reawakened” when the cell revs up its metabolic machinery because of the reparative process. An interesting part of these nucleoli is that they have “roadways” that connect the nucleolar proteins/ rRNA to the cytoplasm where they can be exported for mRNA synthesis.
Surely, these “roadways” are part of the three-dimensional macromolecule cross-linked network that is held rigidly in place when the cells are fixed. Another interesting part of this “nucleolar to cytoplasm roadway” is the size of the molecules that can easily pass through it. It has been documented that proteins up to 2000kDa in size can pass through the roadway.
Such proteins are huge in comparison to the proteins we use in immunohistochemical and in situ hybridization. As you may recall, the antibodies we use are about 100kDa in size, streptavidin is 60kDa, peroxidase is 150kDa, and so on. Although no doubt formalin fixation, via extensive cross-linking, creates pores that restrict the size of macromolecules, it is good to remember that the living cell is capable of trafficking molecules much larger than the key reagents we use with immunohistochemistry and in situ hybridization.
The bottom line with respect to the histopathology of reparative changes is that it is one of those circumstances (as with cancer cells) in which all the cells start to look the same, regardless of their cell of origin. This is evident from a review of Figure 6-12. Note the similar-looking reactive/reparative cells in the squamous epithelia of the cervix (panel A), the glands of the stomach (panel B), the endothelial cells in the esophagus (panel C), and the endothelial cells/fibroblasts of the stomach by an ulcer (panel D). Also note the strong inflammation associated with each biopsy in the panels where the stroma is evident.
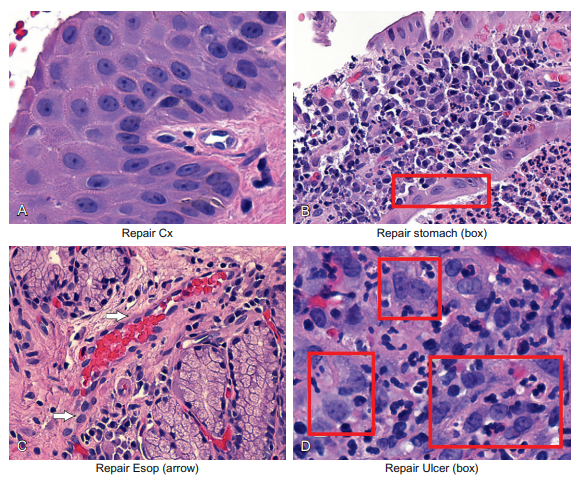
Figure 6-12 Epithelial cells and repair. If epithelial cells are exposed to chronic inflammation, they show typical cellular changes called repair. The cytologic changes will look very similar to stromal cells undergoing chronic or acute inflammation. The cell’s metabolic machinery changes, and this is seen with enlarged, clear nuclei with small nucleoli. The cytoplasm usually remains nondescript. The end result is that the nuclear/cytoplasmic ratio increases. This is seen in panel A for the squamous cells in the cervix, in panel B for the glandular cells of the stomach, in panel C in the endothelial cells in the esophagus, and in panel D in the endothelial cells and fibroblasts around an ulcer.
METAPLASIA
The last term to discuss in this section is metaplasia. It occurs when one cell type “changes” to a different cell type. There are several places in the body where glandular cells form junctions with squamous cells. Logically, the squamous epithelia are in the part of the body nearer the outside surface, and the glandular epithelia are farther away from the outside surface.
Three good examples of this are in the esophagus, the cervix, and at the anal–rectal junction. At these sites, squamous metaplasia is the norm. The term “squamous metaplasia” simply means that the area lined by glandular cells is slowly but surely being replaced by squamous cells. Squamous metaplasia of the cervix is seen in Figure 6-13, panels A and B. Note that the squamous epithelia are overlying the glandular epithelium.
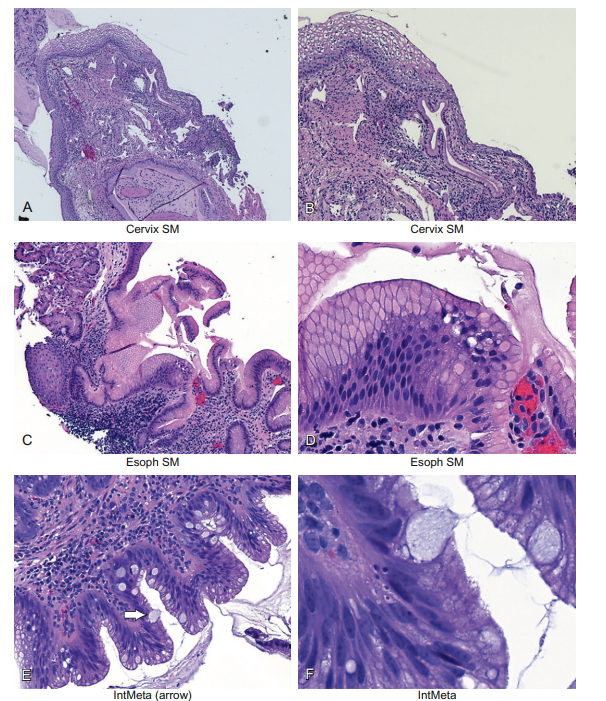
Figure 6-13 Epithelial cells and metaplasia. If epithelial cells are exposed to chronic inflammation, one epithelial cell type often will change to a different cell type. In the cervix and esophagus, in each case the simple glandular epithelia will typically be replaced by stratified squamous epithelia as a “protective” measure. This is seen in panels A and B and, for the esophagus, in panels C and D. Panels E and F show another type of metaplasia of the esophagus called intestinal metaplasia. Here, the lining of the esophagus is replaced by simple columnar epithelia that have large pools of mucus; such cells are called goblet cells and are diagnostic of Barrett’s esophagus (or intestinal metaplasia). People with this condition are at increased risk for adenocarcinoma, likely as a reflection of the chronic inflammatory state and associated repair.
This means that, years before, this area was lined by glandular epithelia, but over time it was replaced by squamous epithelia. The process of squamous metaplasia is very important in the cervix, because the metaplastic cells are especially susceptible to infection by human papillomavirus (HPV) infection. HPV infection is an essential co-factor in cervical cancer, as well as in anorectal cancer. Panels C and D show the same metaplasia, only this time occurring in the esophagus.
Metaplasia reminds us how much “plasticity” cells have (as does repair), being able, under the correct conditions, to change into other cell types. There are other types of metaplasia. There are many different types of glandular cells. Some make mucus, others have cilia, and others are called goblet cells, and so on. Specific types of glandular cells can become other types of glandular cells. For example, in the esophagus the mucus-producing glandular cell can become a goblet cell. Since the goblet cell is commonly found lower in the intestine, this is called intestinal metaplasia.
An example of intestinal metaplasia is seen in Figure 6-13, panels E and F. The “typical” glandular cells of the esophagus are marked by the small arrow, and the goblet cells by the large arrow. The diagnosis of intestinal metaplasia of the esophagus much increases the risk of adenocarcinoma at this site, again most likely reflecting the chronic inflammation at this site. This disease process (chronic inflammation with intestinal metaplasia) in the esophagus is called Barrett’s esophagus.
SPECIALIZED STROMAL CELLS
Metaplasia reminded me of the amazing ability of cells to change their appearance in response to a variety of factors, such as inflammation. Still, when it comes to cells being able to change their appearance and function, it is hard to beat the stromal cell. My intention in this chapter is to give you the histologic foundation to be able to identify the cell types that are found in most human and formalin-fixed, paraffinembedded tissues from other animals. Of course, some of you might be working with specific tissue types such as skeletal muscle or brain. This section deals with some of these other specific tissue types.
The basic stromal cell is the fibroblast. It is long and spindly in appearance. It has relatively little cytoplasm. The reason is that most of its product is secreted into the stroma, where it forms the main skeleton and support of the tissue. Of course, this support protein is collagen. The endometrium shows how the fibroblast has evolved to serve very different functions. Endometrial stroma is highly cellular and consists of cells that are round with little cytoplasm, at least during the proliferative phase of the menstrual cycle.
However, at the second stage of this cycle, the endometrial stroma changes dramatically. The stromal cells show very abundant cytoplasm as they fill with proteins that would help the fertilized egg implant and grow in case of a pregnancy. These enlarged stromal cells are called predecidual cells and can be seen in Figure 6-14, panel B. Of course, stromal cells can show much more dramatic appearances than predecidua. Panels C and D show stromal cells that are secreting large amounts of protein (mostly collagen), which appears dark pink (large arrow). Note that some of the collagen is blue (small arrow), which reflects partial calcification of the collagen. The latter process greatly strengthens the collagen and is the basic extracellular material of bone. Panels C and D reflect bony tissue present in the region of the mandible. Actually, at times fibroblasts can actually grow bone de novo in a process called osseous metaplasia. In panels C and D, we are actually seeing part of the mandibular bone.
Other specialized types of stromal cells include striated muscle cells, as seen in skeletal muscle and cardiac tissue, and smooth muscle cells, which line all large blood vessels, the uterus, bladder, gallbladder, and the intestine, since each site needs to be able to alter its diameter for blood pressure control and peristalsis, respectively. Stromal cells can also be storage cells; large amounts of fat stored in such cells produces the adipose cell. Cartilage is made up of cells that secrete a certain type of avascular matrix that helps give tissue both support and the fluidity to move; thus, it is common in joints. In the central nervous system, we see, besides the ubiquitous endothelial cells/blood vessels, three very specialized cell types:
1. The neuron, which shows very large cells with prominent nucleoli. Indeed, these cells are so metabolically active, having to make the neurotransmitters that may have to be able to move down many centimeters of axons, that we may see the blue-colored RNA molecules in the cytoplasm, where they are called Nissel bodies.
2. The astrocytes, which are smaller round cells that form the main support of the brain and spinal cord via their interlacing processes.
3. The microglial cells, which are simply the macrophage that is an endogenous cell in the brain.
Final mention should be made of the melanocyte. This specialized cell comes from a specific part of the embryo called the neural crest. This cell produces a specific and unique chemical called melanin, which is derived from the amino acid tyrosine. Melanin is able to absorb UV radiation and transform it into heat. This protects the DNA in the cells from UV-related damage and mutagenesis. Needless to say, this explains why melanin-producing cells are in the skin, and there is a strong inverse relationship between the number of melanocytes in the skin and the susceptibility to skin cancer. Melanin also gives hair its color. Finally, melanin is important when performing immunohistochemistry or in situ hybridization because the brown pigment may give us a false-positive reaction (unless we recognize the melanin) if we use DAB as the chromogen.
'The Basics of Histologic Interpretatio' 카테고리의 다른 글
| The Basics of Histologic Interpretations of Tissues (0) | 2024.11.30 |
|---|